CANGRELOR
- CAS NO.:163706-06-7
- Empirical Formula: C17H25Cl2F3N5O12P3S2
- Molecular Weight: 776.36
- MDL number: MFCD09837758
- EINECS: 1592732-453-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2022-12-21 16:56:50
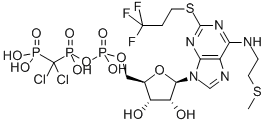
What is CANGRELOR?
The Uses of CANGRELOR
Cangrelor is a P2Y12 inhibitor used as an antiplatelet drug for intravenous application.
The Uses of CANGRELOR
Anti-platelet agent.
Indications
For use as an adjunct to percutaneous coronary intervention (PCI) for reducing the risk of periprocedural myocardial infarction (MI), repeat coronary revascularization, and stent thrombosis (ST) in patients in who have not been treated with a P2Y12 platelet inhibitor and are not being given a glycoprotein IIb/IIIa inhibitor.
Background
Cangrelor is an intravenous, direct-acting, reversible P2Y12 inhibitor for patients undergoing percutaneous coronary intervention (PCI) who have not been yet treated by oral P2Y12 inhibitors. An advantage Cangrelor provides over oral P2Y12 inhibitors (such as prasugrel, ticagrelor, and clopidogrel) is that it is an active drug not requiring metabolic conversion therefore providing a rapid onset and offset of action. Cangrelor was approved by the FDA in June 2015 for intravenous application.
Definition
ChEBI: A nucleoside triphosphate analogue that is 5'-O-[({[dichloro(phosphono)methyl](hydroxy)phosphoryl}oxy)(hydroxy)phosphoryl]adenosine carrying additional 2-(methylsulfanyl)ethyl and (3,3,3-trifluoropropyl)sulfanyl substituents at positions N6 and C2 respectively. Used (in the form of its tetrasodium salt) as an intravenous antiplatelet drug that prevents formation of harmful blood clots in the coronary arteries.
Clinical Use
Direct P2Y12 platelet receptor antagonist:
Antiplatelet for patients undergoing a PCI
Metabolism
Cangrelor is deactivated rapidly in the circulation by dephosphorylation to its primary metabolite, a nucleoside, which has negligible anti-platelet activity. Cangrelor's metabolism is independent of hepatic function and it does not interfere with other drugs metabolized by hepatic enzymes.
Metabolism
Cangrelor is deactivated rapidly in the plasma by dephosphorylation to form its main metabolite, a nucleoside. Following a 2 micrograms/kg/min infusion of [3 H] cangrelor, 58% was found in urine and the remaining 35% was found in faeces, presumably following biliary excretion.
Properties of CANGRELOR
| Boiling point: | 979.0±75.0 °C(Predicted) |
| Density | 2.08 |
| pka | 0.82±0.50(Predicted) |
Safety information for CANGRELOR
Computed Descriptors for CANGRELOR
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


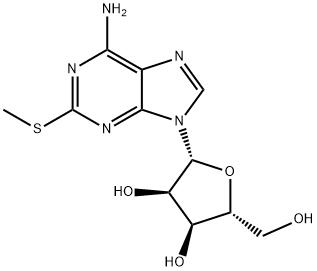
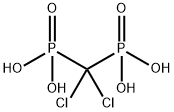
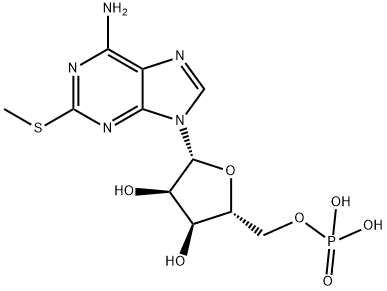
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1