Calcium carbide
- CAS NO.:75-20-7
- Empirical Formula: C2Ca
- Molecular Weight: 64.1
- MDL number: MFCD00010905
- EINECS: 200-848-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
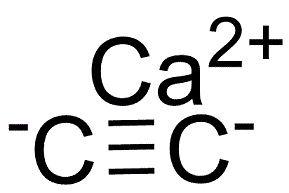
What is Calcium carbide?
Description
Calcium carbide,is a binary salt. It is a grayish-black hard solid that reacts with water to produce acetylene gas, a solid corrosive that is calcium hydroxide, and release heat.
The Uses of Calcium carbide
Calcium carbide is used as a desulfurizer, dehydrant of steel, fuel in steel making, powerful deoxidizer and as a source of acetylene gas. It is used as a starting material for the preparation of calcium cyanamide, ethylene, chloroprene rubber, acetic acid, dicyandiamide and cyanide acetate. Further, it is involved in the reduction of copper sulfide to metallic copper.
Potential Exposure
Those involved in the manufacture andhandling of carbide and the generation of acetylene.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seekmedical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.Medical observation is recommended for 24-48 h afterbreathing overexposure, as pulmonary edema may bedelayed. As first aid for pulmonary edema, a doctor orauthorized paramedic may consider administering a corticosteroid spray.
Source
Calcium carbide is an intermediate product in the smelting of calcium–silicon alloy.
Storage
Color Code—Red Stripe: Flammability Hazard:Do not store in the same area as other flammable materials.Color Code—Green: General storage may be used. Prior toworking with calcium carbide you should be trained on itsproper handling and storage. Store in tightly closed containers in a cool, well-ventilated area away from moisture andwithout sprinkler protection and avoid contact with incompatible materials. Use only nonsparking tools and equipment especially when opening and closing containers ofcalcium carbide. Metal containers involving the transfer ofthis chemical should be grounded and bonded. Use explosion-proof electrical equipment in the carbide-handlingarea. Sources of ignition, such as smoking and open flames,are prohibited where this chemical is used, handled, orstored in a manner that could create a potential fireor explosion hazard.
Shipping
The four-digit UN identification number for calcium carbide is 1402. The NFPA 704 designation is health 3, flammability 3, and reactivity 2. The white section at the bottom of the diamond contains a W with a slash through it, indicating water reactivity. It is shipped in metal cans, drums, and specially designed covered bins on railcars and trucks. When shipped and stored, it should be kept in a cool, dry place.
Incompatibilities
Water contact or moist air forms calciumhydroxide and explosive acetylene gas with risk of fire andexplosion. Keep away from acids, oxidizers, hydrogenchloride, methanol, copper salt solutions, lead fluoride,magnesium, selenium, silver nitrate, iron trichloride, tindichloride, sodium peroxide, stannous chloride, sulfur.
Properties of Calcium carbide
| Melting point: | 447°C |
| Boiling point: | 2300°C |
| Density | 2.22 g/mL at 25 °C(lit.) |
| storage temp. | water-free area |
| solubility | reacts with H2O |
| form | pieces |
| color | Gray-black |
| Specific Gravity | 2.22 |
| Water Solubility | hydrolyses |
| Sensitive | Moisture Sensitive |
| Merck | 14,1656 |
| BRN | 3909011 |
| Exposure limits | ACGIH: TWA 2 mg/m3 OSHA: TWA 5 mg/m3 NIOSH: IDLH 25 mg/m3; TWA 2 mg/m3 |
| Dielectric constant | 5.8 - 7.0(0.0℃) |
| Stability: | Stability Reacts violently with water liberating highly flammable gas (acetylene). Do not use water if this material is involved in a fire. Incompatible with moisture, water, strong oxidizing agents, alcohols, hydrogen chloride, magnesium. |
| EPA Substance Registry System | Calcium carbide (75-20-7) |
Safety information for Calcium carbide
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H260:Substances And Mixtures Which, In Contact With Water,Emit Flammable Gases H315:Skin corrosion/irritation H318:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P223:Keep away from any possible contact with water, because of violent reaction and possible flash fire. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P231+P232:Handle under inert gas. Protect from moisture. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Calcium carbide
| InChIKey | UIXRSLJINYRGFQ-UHFFFAOYSA-N |
Calcium carbide manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Calcium carbide CAS 75-20-7View Details
Calcium carbide CAS 75-20-7View Details
75-20-7 -
 Calcium carbide CAS 75-20-7View Details
Calcium carbide CAS 75-20-7View Details
75-20-7 -
 CALCIUM CARBIDE Extra Pure CAS 75-20-7View Details
CALCIUM CARBIDE Extra Pure CAS 75-20-7View Details
75-20-7 -
 Calcium carbide CAS 75-20-7View Details
Calcium carbide CAS 75-20-7View Details
75-20-7 -
 Calcium carbide CAS 75-20-7View Details
Calcium carbide CAS 75-20-7View Details
75-20-7 -
 Powder Calcium Carbide (75-20-7), For Industrial, Packaging Size: 25 kgView Details
Powder Calcium Carbide (75-20-7), For Industrial, Packaging Size: 25 kgView Details
75-20-7 -
 Calcium Carbide, LumpView Details
Calcium Carbide, LumpView Details
75-20-7 -
 Crushed Calcium CarbideView Details
Crushed Calcium CarbideView Details
75-20-7
