Hydroxyapatite
Synonym(s):HAp;Calcium phosphate tribasic;Hydroxylapatite;Tribasic calcium phosphate;Calcium hydroxyphosphate
- CAS NO.:1306-06-5
- Empirical Formula: Ca5HO13P3
- Molecular Weight: 502.31
- MDL number: MFCD00010904
- EINECS: 215-145-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:30
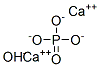
What is Hydroxyapatite?
Description
Calcium hydroxyapatite has a unique structure in
that it is conductive along the hydroxide channels.
OH- ions lie at (1/4/,1/4,1/4) and (3/4/,3/4,3/4) on
the c-axis and charge-carrying protons are responsible
for the observed conductivities in M10(PO4)6(OH)2. The
H+ migration between the electroattractive ion (O2)
to give molecular H2O in matrix channels carries charge
and the resulting conductivity.
The unit cell consists of two triangular prismatic subcells
forming a rhombic prism with vertical sides. There
are two horizontal mirror planes at the OH levels of 1/4
and 3/4 of the c-axis. In addition, there is a center of
inversion exactly in the center of each vertical face of
each subcell.
Description
Hydroxyapatite, sometimes referred to as durapatite, is a calcium phosphate/hydroxide mineral that occurs naturally in “phosphate rock”. It is also present in as much as 70 wt% of human bone.
Although the formula for hydroxyapatite is usually expressed as Ca5(PO4)3OH, it is sometimes given as Ca10(PO4)6OH2 or 3Ca2(PO4)2CaOH2 to indicate that its crystal unit cell consists of two formula weights. In the 3-D image shown above, calcium is represented in green, phosphorus in gray, oxygen in pink, and hydrogen in black.
In addition to natural hydroxyapatite, the commercial substance is frequently synthetic. It was made as long ago as 1873, when British agricultural chemist Robert Warington, Jr., prepared it from calcium nitrate and potassium dihydrogen phosphate. In modern times, it is synthesized via wet chemical precipitation, biomimetic deposition, or electrodeposition. One biosynthetic method that used the bacterium Klebsiella pneumoniae was reported in 2019 by Arivalagan Pugazhendhi, Rathinasamy Subashkumar, and coauthors at institutions in India and Viet Nam.
Natural hydroxyapatite contains impurities, so its main use is as a source of phosphate and subsequently other phosphorus-containing compounds. The much purer synthetic material is used to form artificial teeth and bones or repair natural tissues. Several aspects of hydroxyapatite chemistry and biology are covered in the ScienceDirect page about the molecule.
When you see skeletons hanging about this Halloween week, think of hydroxyapatite!
Chemical properties
white powder
Chemical properties
Tricalcium phosphate is an odorless and tasteless powder that is stable in air. Tribasic calcium phosphate consists of a variable mixture of calcium phosphates having the approximate composition of 10CaO·3P2O5·H2O.
Occurrence
Occurs in nature as the minerals: oxydapatit, voelicherite, whitlockite.
The Uses of Hydroxyapatite
In the 1970s, it was found that sintered calcium hydroxyapatite, (Ca10(PO4)6(OH)2 (abbreviated as CaHap), possessed excellent biocompatibility and nontoxicity with femur and mandible bones. Since then, CaHap has been used as a biomaterial for artificial teeth and bones and as a filler for cements and polymers. Today, bone fillers made of CaHap are widely used in the medical and dental fields. In the 1980s, it was found that sintered CaHap has a good compatibility with skin tissues. Hence, percutaneous devices based on CaHap have been developed and applications have included continuous ambulatory peritoneal dialysis and intravenous hyperalimentation systems, blood pressure measurement and blood access for nutrition. Recently, many researchers have studied the chemistry of apatites, particularly CaHap, and have found various applications, such as artificial teeth and bones, ion exchangers, adsorbents for chromatography to separate proteins and enzymes, catalysts, ionic conductors, temperature and gas sensors, etc.
The Uses of Hydroxyapatite
Prosthetic aid (artificial bone and teeth).
The Uses of Hydroxyapatite
Hydroxyapatite and tricalcium phosphate are bioactive ceramic materials and they find applications as bone grafts, fillers and coating material for metal implants.
Definition
The major constituent of bone and tooth mineral. It is finely divided, crystalline, nonstoichiometric material rich in surface ions (carbonate, magnesium, citrate), which are readily replaced by fluoride ion, thus affording protection to the teeth.
Preparation
The technical product is also known as “bone ash.” Commercial preparation from phosphate rock.
brand name
Alveograf (Sterling Winthrop); Periograf (Sterling Winthrop).
General Description
Bone and tooth implant materials have been prepared from polycrystalline hydroxyapatite. The compressive, flexural, torsional and dynamic torsional strengths of polycrystalline hydroxyapatite were investigated. Electrophoretic deposition of hydroxyapatite on to flat titanium plate material has been studied.
Flammability and Explosibility
Not classified
Pharmaceutical Applications
Modified hydroxylapatite, also frequently called hydroxyapatite and better known as bone mineral, makes up ~50% of our bones. Hydroxylapatite is a natural form of the mineral calcium apatite, whose formula is usually denoted as Ca10(PO4)6(OH)2. Modifications of hydroxylapatite can also be found in the teeth, and a chemically identical substance is often used as filler for replacement of bones, and so on. Nevertheless, despite similar or identical chemical compositions, the response of the body to these compounds can be quite different.
Properties of Hydroxyapatite
| Melting point: | 1100 °C(lit.) |
| Density | 3.076 g/cm3(Temp: 18 °C) |
| storage temp. | 2-8°C |
| solubility | H2O: 0.3 mg/mL, clear, colorless |
| appearance | white crystals or powdera |
| form | solid |
| color | White |
| Water Solubility | insoluble H2O [MER06] |
| Merck | 13,3500 |
| CAS DataBase Reference | 1306-06-5(CAS DataBase Reference) |
| EPA Substance Registry System | Hydroxylapatite (Ca5(OH)(PO4)3) (1306-06-5) |
Safety information for Hydroxyapatite
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for Hydroxyapatite
Hydroxyapatite manufacturer
Krishna Chemicals
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

You may like
-
 Hydroxylapatite ~ 25% solids suspension (10g solids/40ml) in 0.001M CAS 1306-06-5View Details
Hydroxylapatite ~ 25% solids suspension (10g solids/40ml) in 0.001M CAS 1306-06-5View Details
1306-06-5 -
 Hydroxylapatite >24% (Calcium) CAS 1306-06-5View Details
Hydroxylapatite >24% (Calcium) CAS 1306-06-5View Details
1306-06-5 -
 Hydroxyapatite CAS 1306-06-5View Details
Hydroxyapatite CAS 1306-06-5View Details
1306-06-5 -
 Hydroxyapatite Nanoparticles/ NanopowderView Details
Hydroxyapatite Nanoparticles/ NanopowderView Details
1306-06-5 -
 Tricalcium Phosphate PowderView Details
Tricalcium Phosphate PowderView Details
7758-87-4 -
 Tri Calcium Phosphate IP BP USP FOOD, 50 kg bagView Details
Tri Calcium Phosphate IP BP USP FOOD, 50 kg bagView Details
7758-87-4 -
 Tricalcium Phosphate Powder, Laboratory Grade, 25 KgView Details
Tricalcium Phosphate Powder, Laboratory Grade, 25 KgView Details
1306-06-5 -
 Tricalcium PhosphateView Details
Tricalcium PhosphateView Details
7758-23-8
