Cadmium sulfide
Synonym(s):Cadmium monosulfide;Cadmium sulfide yellow
- CAS NO.:1306-23-6
- Empirical Formula: CdS
- Molecular Weight: 144.48
- MDL number: MFCD00010922
- EINECS: 215-147-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
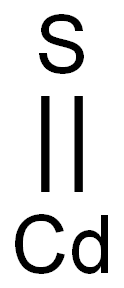
What is Cadmium sulfide?
Description
Cadmium sulfide is an odorless, crystalline,lemon yellow to orange solid. Molecular weight=144.48;Specific gravity (H2O:1)=4.8; Sublimation point=978℃.Insoluble in water.
Chemical properties
yellow to orange crystalline powder
Chemical properties
Cadmium sulfide is an odorless, crystalline, lemon yellow to orange solid.
Physical properties
Yellow to orange crystal; occurs as two polymorphs, hexagonal alpha form and cubic beta form; exhibits stable wurtzite structure at lower temperature, and zinc blende type structure at higher temperatures; the beta form converts to alpha form when heated at 750°C in sulfur atmosphere; sublimes at 980°C; practically insoluble in water (1.3 mg/L at 20°C); Ksp 3.6x10-29; dissolves in dilute mineral acids on heating or concentrated acids at ordinary temperatures (decomposes with liberation of H2S).
Occurrence
Cadmium sulfide occurs in nature as the mineral greenoktite. The compound is widely used in pigments for paints, baking enamels, ceramics and plastics. It imparts bright yellow to maroon, with strong retention of color and resistance to alkalis. It also is used in inks, phosphors, and fluorescent screens. Other applications of this compound are in photovoltaic and solar cells (for converting solar energy to electrical energy), photoconductors (in xerography), thin film transistors and diodes, rectifiers, scintillation counters, pyrotechnics, and smoke detectors.
The Uses of Cadmium sulfide
cadmium sulfide is used as a colorant for paints and rubber; cadmium acetate is used in the production of craftware.
The Uses of Cadmium sulfide
Used in the production of solar cells where it is used as a buffer layer in the manufacture of CIGS (Copper -Indium-Gallium-Selenide) solar cells
The Uses of Cadmium sulfide
As a pigment being fast to light and not affected by H2S; color for soaps; coloring glass yellow; coloring textiles, paper, rubber; in printing inks, ceramic glazes, fireworks; in phosphors and fluorescent screens; in scintillation counters, semiconductors, photoconductors.
What are the applications of Application
Cadmium sulfide is an inorganic compound used as a pigment and in the manufacturing of photoresistors
Definition
A native cadmium sulfide containing 77.7% cadmium. Ore of cadmium
Definition
Greenockite: a mineral form ofcadmium sulphide, CdS.
Production Methods
Cadmium sulfide may be prepared by the reaction between hydrogen sulfide and cadmiumvapor at 800 Cor by heating a mixture of cadmium or cadmium oxide with sulfur.
Preparation
Cadmium sulfide may be prepared by precipitation from an aqueous solution of its soluble salts such as cadmium chloride or cadmium nitrate by passing hydrogen sulfide. The reactions may be carried out in acidic, neutral or alkaline solutions using various cadmium salts to obtain different crystal modifications as shown in the table below.
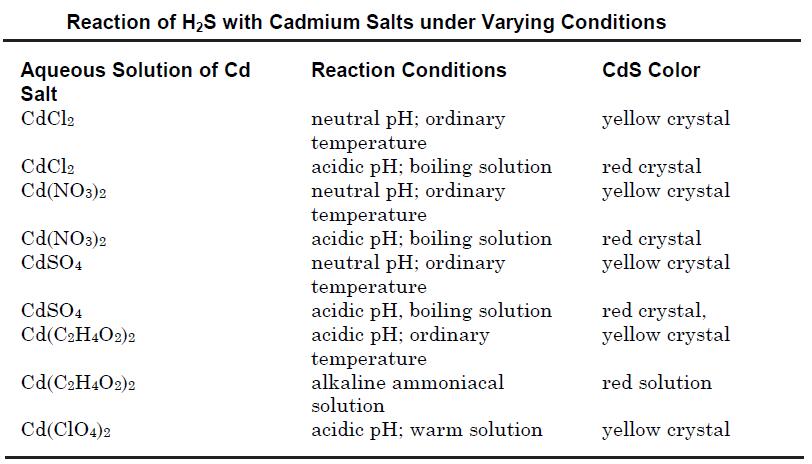
Cadmium sulfide also may be obtained by treatment of sodium or other alkali metal sulfide solution with that of a soluble cadmium salt. The compound also may be prepared by heating a mixture of cadmium or its oxide with sulfur at 800°C; or by the reaction of H2S with cadmium vapor at 800°C.
General Description
Natural occurrence: hawleyite (structural type of sphalerite) and greenockite (structural type of wurtzite)
Hazard
A confirmed carcinogen, highly toxic. See cadmium.
Safety Profile
Confirmed human carcinogen with experimental carcinogenic and tumorigenic data. Moderately toxic by ingestion and inhalation. Human mutation data reported. When heated to decomposition it emits very toxic fumes of Cd and SOx. See also CADMIUM COMPOUNDS and SULFIDES
Potential Exposure
Used in pigments; as an active ingredient in dandruff shampoos; making photoconductors, solar cells, and other electronic components.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for atleast 15 min, occasionally lifting upper and lower lids.Seek medical attention immediately. If this chemicalcontacts the skin, remove contaminated clothing and washimmediately with soap and water. Seek medical attentionimmediately. If this chemical has been inhaled, removefrom exposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing hasstopped and CPR if heart action has stopped. Transferpromptly to a medical facility. When this chemical hasbeen swallowed, get medical attention. Give large quantitiesof water and induce vomiting. Do not make an unconsciousperson vomit. Medical observation is recommended for24-48 h after breathing overexposure, as pulmonary edemamay be delayed. As first aid for pulmonary edema, a doctoror authorized paramedic may consider administering a corticosteroid spray.Note to physician: In case of fume inhalation, treat pulmonary edema. Give prednisone or other corticosteroid orallyto reduce tissue response to fume. Positive-pressureventilation may be necessary. Treat metal fume fever withbed rest, analgesics, and antipyretics. The symptoms ofmetal fume fever may be delayed for 4-12 h followingexposure: it may last less than 36 h.Note to physician: For severe poisoning do not useBAL [British Anti-Lewisite, dimercaprol, dithiopropanol(C3H8OS2)] as it is contraindicated or ineffective inpoisoning from cadmium.
Solubility in water
Cadmium sulfide is soluble in water, with a solubility of 13×10-5 g/100 ml H2O (18℃). CdS is soluble in acid.
storage
Color Code—Blue: Health Hazard: Store in asecure poison location. Prior to working with Cadmium sulfide, you should be trained on its proper handling and storage. A regulated, marked area should be established wherethis chemical is handled, used, or stored in compliance withOSHA Standard 1910.1045. Store in tightly closed containers in a cool, dark, well-ventilated area away from oxidizersand metals, strong acids, water or moisture, and otherincompatible materials listed above.
Shipping
UN2570 Cadmium compounds, Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required.
Structure and conformation
Cadmium sulfide takes two types of structure, zinc blend and wurtzite structures.
Cubic system that has a zinc-blend structure, with a lattice constant of a=0.582 nm and Cd– S=0.252 nm.
Hexagonal system that has a wurtzite structure with lattice constants of a=0.4136 nm, c=0.6713 nm and c/a=1.624, Cd–S=0.252 nm.
Incompatibilities
Contact with water or moisture releases poisonous hydrogen sulfide gas. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause
Waste Disposal
Use a licensed professional waste disposal service to dispose of this material. All federal, state, and local environmental regulations must be observed. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform to EPA regulations governing storage, transportation, treatment, and waste disposa
Properties of Cadmium sulfide
| Melting point: | 980°C (subl.) |
| Boiling point: | 980°C (estimate) |
| Density | 4.82 g/mL at 25 °C(lit.) |
| Flash point: | 4℃ |
| storage temp. | 2-8°C |
| solubility | Soluble in acid, very slightly soluble in ammonium hydroxide. |
| form | powder |
| color | Yellow to orange |
| Specific Gravity | 4.82 |
| Water Solubility | Insoluble |
| Crystal Structure | Cubic, Sphalerite Structure - Space Group F(-4)3m |
| Merck | 14,1628 |
| Solubility Product Constant (Ksp) | pKsp: 26.1 |
| Exposure limits | ACGIH: TWA 0.01 mg/m3; TWA 0.002 mg/m3 NIOSH: IDLH 9 mg/m3 |
| CAS DataBase Reference | 1306-23-6(CAS DataBase Reference) |
| EPA Substance Registry System | Cadmium sulfide (1306-23-6) |
Safety information for Cadmium sulfide
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H341:Germ cell mutagenicity H350:Carcinogenicity H372:Specific target organ toxicity, repeated exposure H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Cadmium sulfide
Cadmium sulfide manufacturer
ARRAKIS INDUSTRIES LLP
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid Fmoc-Val-Cit-PAB DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonateRelated products of tetrahydrofuran

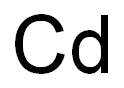

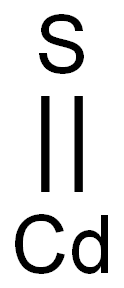
You may like
-
 Cadmium sulfide CAS 1306-23-6View Details
Cadmium sulfide CAS 1306-23-6View Details
1306-23-6 -
 Cadmium sulfide CAS 1306-23-6View Details
Cadmium sulfide CAS 1306-23-6View Details
1306-23-6 -
 Cadmium sulfide CAS 1306-23-6View Details
Cadmium sulfide CAS 1306-23-6View Details
1306-23-6 -
 Cadmium sulfide CAS 1306-23-6View Details
Cadmium sulfide CAS 1306-23-6View Details
1306-23-6 -
 Cadmium sulphide CAS 1306-23-6View Details
Cadmium sulphide CAS 1306-23-6View Details
1306-23-6 -
 Cadmium sulphide CAS 1306-23-6View Details
Cadmium sulphide CAS 1306-23-6View Details
1306-23-6 -
 Cadmium sulfide CAS 1306-23-6View Details
Cadmium sulfide CAS 1306-23-6View Details
1306-23-6 -
 CADMIUM SULFIDE CHEMICAL CAS 1306-23-6View Details
CADMIUM SULFIDE CHEMICAL CAS 1306-23-6View Details
1306-23-6