Cadmium bromide
- CAS NO.:7789-42-6
- Empirical Formula: Br2Cd
- Molecular Weight: 272.22
- MDL number: MFCD00015996
- EINECS: 232-165-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
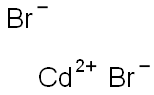
What is Cadmium bromide?
Description
admium bromide is a white to yellowishcrystalline powder. Molecular weight=272.22; BoilingCadmium bromide 505point=863℃; Freezing/Melting point=567℃. HazardIdentification (based on NFPA-704 M Rating System):Health 3, Flammability 0, Reactivity 0. Soluble in water.
Chemical properties
White to yellowish crysatlline powder
Chemical properties
Cadmium bromide is a white to yellowish crystalline powder.
Physical properties
White to yellowish powder or flakes; hexagonal crystal system; hygroscopic; density 5.192g/cm3; melts at 568°C; vaporizes at 844°C; soluble in water, alcohol, ether, acetone, and liquid ammonia.
The Uses of Cadmium bromide
Made by heating cadmium to redness in bromine vapor. The yellowish crystalline powder is soluble in water and alcohol and is slightly soluble in ether. The crystals are deliquescent and must be kept in a well-stoppered bottle. Like its iodide counterpart, cadmium bromide was used in collodion in conjunction with an iodide of either ammonium or potassium.
The Uses of Cadmium bromide
Process engraving, lithography and photography
The Uses of Cadmium bromide
In photography, process engraving, and lithography.
The Uses of Cadmium bromide
This compound is used in photography, engraving, and lithography. The other halogen elements also combine with cadmium in a similar ionic reaction as with bromine.
Preparation
Cadmium bromide is prepared by heating cadmium with bromine vapor. Also the compound can be prepared by the treatment of dry cadmium acetate with glacial acetic acid and acetyl bromide. Alternatively, it may be obtained by dissolving cadmium or cadmium oxide in hydrobromic acid and evaporating the solution to dryness under helium in an inert atmosphere.
General Description
Odorless white solid. Mixes with water.
Air & Water Reactions
Water soluble.
Reactivity Profile
CADMIUM BROMIDE has weak oxidizing or reducing powers. Redox reactions can however still occur. The majority of compounds in this class are slightly soluble or insoluble in water. If soluble in water, then the solutions are usually neither strongly acidic nor strongly basic. These compounds are not water-reactive.
Health Hazard
Inhalation causes coughing, sneezing, symptoms of lung damage. Ingestion produces severe toxic symptoms; both kidney and liver injuries may occur. Contact with eyes causes irritation.
Fire Hazard
Special Hazards of Combustion Products: Toxic cadmium oxide fumes may form in fires.
Safety Profile
Confirmed human carcinogen. When heated to decomposition it emits toxic fumes of Cd and Br-.
Potential Exposure
Cadmium bromide is used in photography, engraving, and lithography
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seekmedical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions,506 Cadmium bromideincluding resuscitation mask) if breathing has stopped andCPR if heart action has stopped. Transfer promptly to amedical facility. When this chemical has been swallowed,get medical attention. Give large quantities of water andinduce vomiting. Do not make an unconscious personvomit. Medical observation is recommended for 24-48 hafter breathing overexposure, as pulmonary edema may bedelayed. As first aid for pulmonary edema, a doctor orauthorized paramedic may consider administering a corticosteroid spray.Note to physician: For severe poisoning do not use BAL[British Anti-Lewisite, dimercaprol, dithiopropanol(C3H8OS2)] as it is contraindicated or ineffective in poisoningfrom cadmium.
storage
Color Code—Blue: Health Hazard: Store in asecure poison location. Prior to working with cadmiumbromide you should be trained on its proper handling andstorage. Cadmium bromide must be stored to avoid contactwith Potassium, since violent reactions occur. A regulated,marked area should be established where this chemical ishandled, used, or stored in compliance with OSHAStandard 1910.1045.
Shipping
UN 2570 Cadmium compounds, Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required
Purification Methods
Crystallise it from water (0.6mL/g) between 100o and 0o, and dry it at 110o. It forms the monohydrate below 36o and the tetrahydrate above 36o. [Wagenknecht & Juza in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol II p 1096 1965.]
Properties of Cadmium bromide
| Melting point: | 567°C |
| Boiling point: | 863°C |
| Density | 5,192 g/cm3 |
| Flash point: | 863°C |
| solubility | Soluble in alcohol, ether, acetone and liquid ammonia. |
| form | beads |
| color | White to pale yellow |
| Specific Gravity | 5.192 |
| Water Solubility | Freely soluble in water, alcohol. Slightly soluble in acetone and ether. |
| Sensitive | Hygroscopic |
| Merck | 14,1615 |
| Exposure limits | ACGIH: TWA 0.01 mg/m3; TWA 0.002 mg/m3 NIOSH: IDLH 9 mg/m3 |
| Stability: | Stable. |
| CAS DataBase Reference | 7789-42-6(CAS DataBase Reference) |
| EPA Substance Registry System | Cadmium bromide (7789-42-6) |
Safety information for Cadmium bromide
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H312:Acute toxicity,dermal H332:Acute toxicity,inhalation H400:Hazardous to the aquatic environment, acute hazard H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P321:Specific treatment (see … on this label). P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. |
Computed Descriptors for Cadmium bromide
Cadmium bromide manufacturer
Axiom Chemicals Pvt Ltd (Axiom Corporation)
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one N-octanoyl benzotriazole 3,4-Dibenzyloxybenzaldehyde 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 4-HYDROXY BENZYL ALCOHOL 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

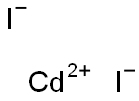


You may like
-
 7789-42-6 Cadmium bromide anhydrous 98%View Details
7789-42-6 Cadmium bromide anhydrous 98%View Details
7789-42-6 -
 Cadmium bromide, Ultra dry CAS 7789-42-6View Details
Cadmium bromide, Ultra dry CAS 7789-42-6View Details
7789-42-6 -
 Cadmium bromide, Ultra dry CAS 7789-42-6View Details
Cadmium bromide, Ultra dry CAS 7789-42-6View Details
7789-42-6 -
 Cadmium bromide CAS 7789-42-6View Details
Cadmium bromide CAS 7789-42-6View Details
7789-42-6 -
 Cadmium bromide CAS 7789-42-6View Details
Cadmium bromide CAS 7789-42-6View Details
7789-42-6 -
 Cadmium bromide CAS 7789-42-6View Details
Cadmium bromide CAS 7789-42-6View Details
7789-42-6 -
 Reduced Iron Powder 99.8% MaxView Details
Reduced Iron Powder 99.8% MaxView Details
7439-89-6 -
 Electrolytic Iron Powder 7439-89-6 99.8% MaxView Details
Electrolytic Iron Powder 7439-89-6 99.8% MaxView Details
7439-89-6