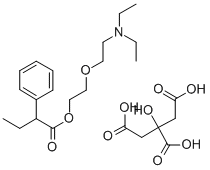
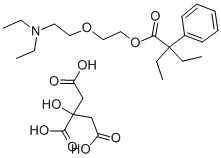
Butamyrate
- CAS NO.:18109-80-3
- Empirical Formula: C18H29NO3
- Molecular Weight: 307.431
- MDL number: MFCD00827785
- EINECS: 242-005-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-28 23:16:16
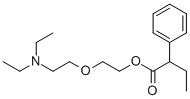
What is Butamyrate?
Originator
Sinecod,Hommel,Switz.,1967
The Uses of Butamyrate
Antitussive.
Definition
ChEBI: Butamirate is an alkylbenzene.
Manufacturing Process
18.2 grams of α-phenylbutyric acid chloride are dissolved in 25 ml of toluene.
To this solution, there is slowly added a solution of 16.1 grams of
diethylaminoethoxyethanol in 25 ml of toluene, the reaction mixture thereby
becoming hot. It is then heated for 8 hr under reflux. The reaction mixture,
after cooling, is carefully poured onto 75 grams of ice and made alkaline with
dilute ammonia. After thorough shaking of the solution, the toluene layer is
removed and washed until neutral with water. The toluene solution is treated
with carbon and dried over sodium sulfate. The toluene is distilled off from the
filtered solution.
The residue is α-phenylbutyric acid diethylaminoethoxyethyl ester. The basic
ester is purified by distillation in a high vacuum. 10 grams of ester are added
to a solution of 7 grams of citric acid in 30 ml of warm acetone. After standing
for some time, the citrate of the ester crystallizes out. After suction filtration and washing with acetone the ester citrate is recrystallized from acetone. The
melting point of the citrate is 75°C.
Therapeutic Function
Antitussive
Properties of Butamyrate
| Melting point: | <25 °C |
| Boiling point: | bp1 140-155° |
| Density | 1.007±0.06 g/cm3(Predicted) |
| storage temp. | -20°C Freezer |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | Oil |
| pka | 9.68±0.25(Predicted) |
| color | Clear Colourless to Dark Yellow |
Safety information for Butamyrate
Computed Descriptors for Butamyrate
Butamyrate manufacturer
Sai Tech Pharamceuticals Pvt Ltd
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 Butamirate 98%View Details
Butamirate 98%View Details
18109-80-3 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1