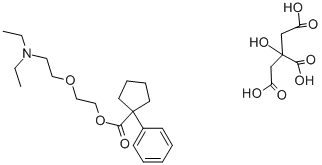
oxeladin
- CAS NO.:468-61-1
- Empirical Formula: C20H33NO3
- Molecular Weight: 335.48
- MDL number: MFCD00242637
- EINECS: 207-412-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-04 17:34:39
What is oxeladin?
Originator
Silopentol,Schulte,W. Germany ,1970
The Uses of oxeladin
Oxeladin can be used in ex vivo modeling of drug efficacy in rare metastatic urachal carcinoma.
Background
Withdrawn from the Canadian, US, and UK markets in 1976 due to carcinogenicity.
Definition
ChEBI: Oxeladin is an alkylbenzene.
Manufacturing Process
Preparation of Diethylphenylacetonitrile: 25 grams of sodium was dissolved in 300 ml liquid ammonia containing 0.3 gram ferric chloride and 59 grams phenylacetonitrile was added slowly with stirring. After about 15 minutes a cooled solution of 80 grams of ethyl chloride in 200 ml dry ether was added and the mixture stirred for 1 hour. The ammonia was then allowed to evaporate, water added and the ether layer separated, dried, concentrated and the residual oil distilled in vacuo to yield diethylphenylacetonitrile as an oil, BP 85°C/1 mm.
Preparation of Diethylphenylacetic Acid: 46 grams of the foregoing nitrile was added to 140 ml ethylene glycol containing 36 grams potassium hydroxide and the mixture refluxed with stirring for about 20 hours. The mixture was diluted with water, extracted with light petroleum (BP 60° to 80°C) to remove traces of impurities and then acidified to yield diethylphenylacetic acid which was recrystallized from dilute ethanol (40% v/v ethanol in water).
Preparation of 2-(β-Chloroethoxy)Ethyl Diethylphenylacetate: 19.2 grams of the foregoing acid was added to a solution of 4 grams of sodium hydroxide in 40 ml ethylene glycol. 28.6 grams β,β'-dichlorodiethyl ether was added and the mixture refluxed for 1 hour. After removal of solvent under reduced pressure, 150 ml water was added to the residue and the product extracted with ether. The ethereal solution was dried, concentrated and the residue distilled in vacuo to yield the product as an oil, BP 140°C/0.7 mm.
Preparation of 2-(β-Diethylaminoethoxy)Ethyl Diethylphenylacetate: A mixture of 21 grams of 2-(β-chloroethoxy)ethyl diethylphenylacetate and 14 grams diethylamine was heated under pressure in a sealed tube at 140°C for 5 hours. After cooling, the mixture was dissolved in dilute hydrochloric acid and extracted with ether to remove traces of neutral impurities. The acid layer was then made alkaline with 10% w/v sodium hydroxide solution with cooling, and re-extracted with two portions of ether. The ether extract was dried, the ether distilled off and the residue distilled in vacuo to yield the product as an oil, BP 140°C/0.1 mm.
Therapeutic Function
Antitussive
Metabolism
Not Available
Properties of oxeladin
| Melting point: | 25°C |
| Boiling point: | bp0.5 150°; bp0.1 140° |
| Density | 1.0720 (rough estimate) |
| refractive index | 1.5614 (estimate) |
Safety information for oxeladin
Computed Descriptors for oxeladin
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1