Brigatinib
- CAS NO.:1197953-54-0
- Empirical Formula: C29H39ClN7O2P
- Molecular Weight: 584.09
- MDL number: MFCD29472221
- EINECS: 1592732-453-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-11 17:37:59
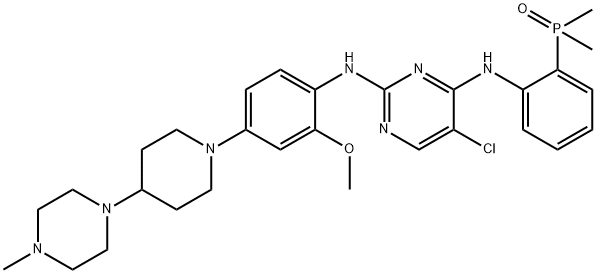
What is Brigatinib?
Absorption
Administration of brigatinib at a concentration of 90 mg generates a Cmax of 552 ng/ml and AUC of 8165 ng h/ml while the administration of 180 mg presents a Cmax of 1452 ng/ml and AUC of 20276 ng h/ml. It has a dose proportional exposure with an accumulation ratio on the range of 1.9 to 2.4. Following oral administration of brigatinib, the Tmax is presented in a range from 1 to 4 hours. Consumption of a high-fat meal compared to overnight fasting reduces Cmax by 13% without presenting an effect on AUC.
Toxicity
Treatment with brigatinib generated not mutagenic chromosomal damage. Testicular toxicity, reported as lower weight of seminal vesicles and prostate gland, testicular tubular degeneration and lower weight as well as reduced size of testes with evidence of hypoespermatogenesis.
Description
Brigatinib is a small molecule inhibitor of both ALK and EGFR. It is used to treat non-small cell lung cancer that has spread to other parts of the body. In preclinical models, “triple-mutant EGFR”-positive cells (activating EGFR mutation/ T790M/C797S) responded to the combination of brigatinib with an anti-EGFR monoclonal antibody, cetuximab or panitumumab. Single-agent activity of brigatinib in a phase I/II trial was only 5%, possibly compounded by low plasma concentrations. Still, the addition of an anti-EGFR monoclonal antibody remains to be clinically tested. The preclinical data with brigatinib and EAI045 in laboratory models with a C797S mutation demonstrate that combination therapies with an anti-EGFR monoclonal antibody may lead to overcoming resistance to osimertinib.
The Uses of Brigatinib
Brigatinib is a tyrosine kinase receptor Mer (MERTK) inhibitor and can be used in combination with epidermal growth factor receptor (EGFR) inhibitors for the treatment of cancer.
Background
Brigatinib, originally named AP26113, is a reversible dual inhibitor of anaplastic lymphoma kinase (ALK) and epidermal growth factor receptor (EGFR). It presents selectivity against the mutant forms of EGFR compared to the wild-type. It also exhibits selectivity against 9 different Crizotinib-resistant mutants of the EML4-ALK fusion gene, which is a pivotal player in the transformation of susceptible lung parenchyma. Brigatinib was developed by Ariad Pharmaceuticals, a subsidiary of Takeda Pharmaceutical Company Limited, and FDA-approved on April 28, 2017.
brand name
Alunbrig?
General Description
Class: receptor tyrosine kinase; Treatment: NSCLC; Elimination half-life = 25 h; Protein binding = 90%
Mechanism of action
The anaplastic lymphoma kinase positive, metastatic non-small cell lung cancer (ALK+ NSCLC) represents only 3-5% of the NSCLC cancer cases, but the ALK mutation, overexpression and presence in several oncogenic fusion proteins in solid and hematologic tumours have pointed out the importance as well as its potential as a cancer therapy target. The ALK-related cases of NSCLC are associated with the fusion gene EML4-ALK, which fused the ALK protein with the echinoderm microtubule-associated protein like-4, whose original function is the correct formation of microtubules. The presence of the aberrant fusion protein results in abnormal signaling that provokes increased cell growth, proliferation and survival. Crizotinib is indicated for treating such cases, but the presence of ALK kinase domain mutations confer resistance to the treatment. Thus, brigatinib is indicated for treating patients with ALK+ NSCLC with intolerance to Crizotinib.
Biologiacal activity
Brigitanib inhibits the proliferation and in vitro viability of cells expressing the fusion protein EML4-ALK and 17 crizotinib-resistant ALK mutants. Its action is expanded to cells expressing EGFR deletions, ROS1-L2026M, FLT3-F691L and FLT3-D835Y. Brigitanib presents a dose-dependent inhibition of tumour growth, tumour burden and prolonged survival in mice EML4-ALK xenograft models.
Side Effects
Nausea, decreased appetite, diarrhea, cough, Tiredness and lack of energy, headache, rash, increased blood sugar levels, and increased blood pressure.
Metabolism
Brigatinib is metabolized by CYP2C8 (72.4%) and CYP3A4 (27.6%) in human liver microsomes and hepatocytes. The two major metabolites generated are the N-demethylated form and the cysteine conjugated form. Oral administration of radiolabelled brigatinib showed the systemic presence of 91.5% in the unchanged form and 3.5% of the primary metabolite AP26123. The AUC of AP26123 is less than 10% of the AUC of brigatinib and presented an inhibitory effect 3 fold lower.
References
Zhang et al. (2016), The potent ALK Inhibitor Brigatinib (AP26113) Overcomes Mechanisms of Resistance to First- and Second-Generation ALK Inhibitors in Preclinical Models; Clin. Cancer Res., 22 5527 Ceccon et al. (2013), Crizotinib-resistant NPM-ALK mutants confer differential sensitivity to unrelated Alk inhibitors; Mol. Cancer Res., 11 122 Takahashi et al. (2020), Overcoming resistance by ALK compound mutation (I1171S + G1269A) after sequential treatment of multiple ALK inhibitors in non-small cell lung cancer; Thorac. Cancer, 11 581 Pinto et al. (2020), Clinical consequences of resistance to ALK inhibitors in non-small cell lung cancer; Respir. Med., 14 385
Properties of Brigatinib
| Melting point: | >203°C (dec.) |
| Boiling point: | 781.8±70.0 °C(Predicted) |
| Density | 1.31±0.1 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | Soluble in DMSO (1 mg/ml with warming) |
| form | solid |
| pka | 8.14±0.42(Predicted) |
| color | White to off-white |
| Stability: | Stable for 2 years from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 1 month. |
| CAS DataBase Reference | 1197953-54-0 |
Safety information for Brigatinib
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Brigatinib
| InChIKey | AILRADAXUVEEIR-UHFFFAOYSA-N |
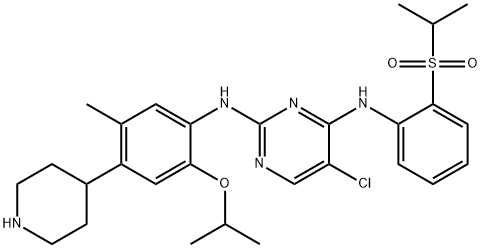
| SMILES | C1(NC2=CC=C(N3CCC(N4CCN(C)CC4)CC3)C=C2OC)=NC=C(Cl)C(NC2=CC=CC=C2P(C)(C)=O)=N1 |
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 1197953-54-0 Brigatinib 98%View Details
1197953-54-0 Brigatinib 98%View Details
1197953-54-0 -
 1197953-54-0 98%View Details
1197953-54-0 98%View Details
1197953-54-0 -
 Brigatinib API 98%View Details
Brigatinib API 98%View Details
1197953-54-0 -
 Brigatinib 95% CAS 1197953-54-0View Details
Brigatinib 95% CAS 1197953-54-0View Details
1197953-54-0 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4
