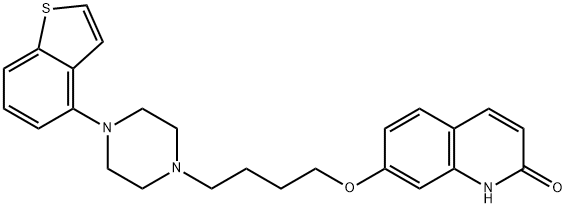
Brexpiprazole
- CAS NO.:913611-97-9
- Empirical Formula: C25H27N3O2S
- Molecular Weight: 433.57
- MDL number: MFCD27987920
- EINECS: 811-628-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-05-29 21:57:13
What is Brexpiprazole?
Absorption
After a single-dose administration, the Tmax was four hours and the absolute oral bioavailability was 95%. Brexpiprazole steady-state concentrations were attained within 10 to 12 days of dosing. After single and multiple once-daily dose administration, the Cmax and AUC increased dose-proportionally.
A high-fat meal did not significantly affect the Cmax or AUC of brexpiprazole.
Toxicity
There is limited information regarding acute toxicity and human overdosage with brexpiprazole. Management of overdose should concentrate on supportive therapy, maintaining an adequate airway, oxygenation and ventilation, and management of symptoms. Close medical supervision and monitoring should continue until the patient recovers. Oral activated charcoal and sorbitol (50 g/240 mL), administered one hour after ingesting oral brexpiprazole, decreased brexpiprazole Cmax and area under the curve (AUC) by approximately 5% to 23% and 31% to 39% respectively; however, there is insufficient information available on the therapeutic potential of activated charcoal in treating an overdose with brexpiprazole. There is no information on the effect of hemodialysis in treating an overdose with brexpiprazole; hemodialysis is unlikely to be useful because brexpiprazole is highly bound to plasma proteins.
Description
Brexpiprazole is a novel antipsychotic drug which serves as a serotonin ® dopamine activity modulator and has demonstrated efficacy as an adjunctive treatment in patients with major depressive disorder (MDD). The drug exhibits a unique pharmacological profile, acting as a partial agonist of serotonin 5-HT1A and dopamine D2 receptors and as a full antagonist of 5-HT2A and noradrenaline α1B/2C receptors, with similar subnanomolar binding affinity. The drug, which was developed by Otsuka and Lundbeck, was approved in 2015 by the FDA for the treatment of schizophrenia and as an adjunctive treatment for depression. Brexpiprazole is widely considered to be a successor to Otsuka’s antipsychotic drug aripiprazole (trade name Abilify) whose patent expired in August 2014.
The Uses of Brexpiprazole
Brexpiprazole is a drug candidate useful in treatment and prevention of mental disorders including CNS disorders.
The Uses of Brexpiprazole
Brexpiprazole is a kind of atypical antipsychotic. It is a dopamine D2 receptor partial agonist. As a novel serotonin-dopamine activity modulator, it can be used for the treatment of schizophrenia, and for the adjunctive treatment for depression. It can also provide efficacy and tolerability over established adjunctive treatments formajor depressive disorder(MDD).Recent study has also suggested it can ameliorate PCP-induced cognitive deficits in mice via 5-HT1A receptors.
Background
Brexpiprazole is an atypical antipsychotic and a novel D2 dopamine and serotonin 1A partial agonist called serotonin-dopamine activity modulator (SDAM). It has a high affinity for serotonin, dopamine and alpha (α)-adrenergic receptors. Although it is structurally similar to aripiprazole, brexpiprazole has different binding affinities for dopamine and serotonin receptors. Compared to aripiprazole, brexpiprazole has less potential for partial agonist-mediated adverse effects such as extrapyramidal symptoms, which is attributed to lower intrinsic activity at the D2 receptor. It also displays stronger antagonism at the 5-HT1A and 5-HT2A receptors.
Brexpiprazole was first approved by the FDA on July 10, 2015. Currently approved for the treatment of depression, schizophrenia, and agitation associated with dementia due to Alzheimer’s disease, brexpiprazole has also been investigated in other psychiatric disorders, such as post-traumatic stress disorder.
Indications
Brexpiprazole is indicated as adjunctive therapy to antidepressants for the treatment of major depressive disorder in adults. It is also indicated for the treatment of schizophrenia in patients 13 years of age and older.
Brexpiprazole is also indicated for the treatment of agitation associated with dementia due to Alzheimer’s disease; however, it is not indicated as an as-needed (“prn”) treatment for this condition.
Definition
ChEBI: Brexpiprazole(913611-97-9) is a N-arylpiperazine. It is a novel D2 dopamine and serotonin 1A partial agonist, called serotonin-dopamine activity modulator (SDAM), and a potent antagonist of serotonin 2A receptors, noradrenergic alpha 1B and 2C receptors. Brexpiprazole is a drug candidate useful in treatment and prevention of mental disorders including CNS disorders.
Pharmacokinetics
Brexpiprazole is an atypical antipsychotic agent used to ameliorate the symptoms of psychiatric conditions, such as cognitive deficits and affective symptoms. Brexpiprazole has affinity (expressed as Ki) for multiple monoaminergic receptors including serotonin 5-HT1A (0.12 nM), 5-HT2A (0.47 nM), 5-HT2B (1.9 nM), 5-HT7 (3.7 nM), dopamine D2 (0.30 nM), D3 (1.1 nM), and noradrenergic α1A (3.8 nM), α1B (0.17 nM), α1D (2.6 nM), and α2C (0.59 nM) receptors. Brexpiprazole acts as a partial agonist at the 5-HT1A, D2, and D3 receptors and as an antagonist at 5-HT2A, 5-HT2B, 5-HT7, α1A, α1B, α1D, and α2C receptors. Brexpiprazole also exhibits affinity for histamine H1 receptor (19 nM) and for muscarinic M1 receptor (67% inhibition at 10 μM).
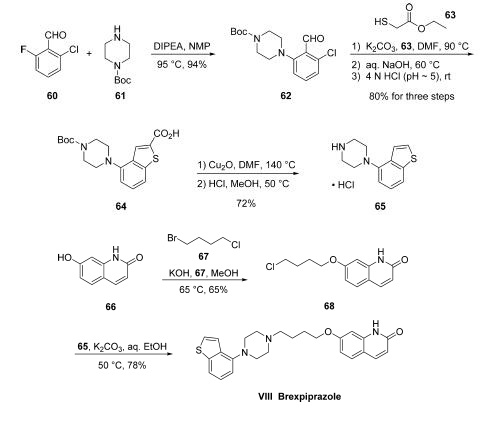
Synthesis
Commercially available fluorobenzaldehyde (60) underwent
a substitution reaction with commercial tert-butyl piperazine-1-
carboxylate (61) under basic conditions to afford the
piperazinyl benzaldehyde 62 in excellent yield. Next, the
construction of the benzothiophene was affected by initial
condensation of thioglycolic acid ethyl ester 63 with ochlorobenzaldehyde
62 under mildly basic conditions at
elevated temperatures. Treatment with aqueous base and
adjustment of pH to roughly 5 through the use of 4 N HCl
furnished the 2-carboxylic acid benzothiophene 64 in 80% yield
across the three-step operation. Next, decarboxylation through
the use of cuprous oxide using conditions slightly modified
from those originally described by Goosen followed by acidic
removal of the Boc protecting group on the terminal piperazine
nitrogen secured the key piperazinyl benzothiophene subunit
65 as the corresponding hydrochloride salt.
The hydroxyquinolone and linker component synthesis
began with alkylation of commercially available quinolone 66
with 1,4-bromochlorobutane (67) under basic conditions to
furnish chloroalkoxyquinolone 68. A subsequent alkylation with
hydrochloride salt 65 using potassium carbonate and warm
aqueous ethanol followed by recrystallizative workup resulted
in clean conversion to brexpiprazole (VIII) in 78% yield from
68.
Enzyme inhibitor
This serotonin-dopamine activity modulator, or SDAM (FW = 433.60 g/mol; CAS 913611-97-9), also known as OPC-34712, Rexulti, 7-{4-[4-(1- benzothiophen-4-yl)piperazin-1-yl]butoxy}quinolin-2(1H)-one, is an atypical antipsychotic drug that acts as a dopamine D2L (Ki = 1.1 nM) and D3 (Ki = 0.3 nM) receptor partial agonist and a partial agonist of 5-HT1A receptors (Ki = 0.12 nM). Brexpiprazole is also an antagonist of the 5-HT2A (Ki = 0.47 nM), 5-HT2B (Ki = 1.9 nM), 5-HT7 (Ki = nM), α1B-adrenergic (Ki = 0.17 nM), α2C-adrenergic (Ki = 0.59 nM), and histamine H1 receptors (Ki = 19 nM). It has negligible affinity for the mACh receptors. Rexulti is an FDA-approved add-on medication for major depressive disorder in adults. See Aripiprazole
Metabolism
According to in vitro studies, brexpiprazole is mainly metabolized by CYP3A4 and CYP2D6. Brexpiprazole and its major metabolite, DM-3411, were the predominant drug moieties in the systemic circulation following single and multiple dose administration. At steady-state, DM-3411 represented 23% to 48% of brexpiprazole exposure (AUC) in plasma. DM-3411 is considered not to be pharmacologically active.
Mode of action
Brexpiprazole's suggested mechanism of action is based on its impact on dopamine and serotonin receptors. As a serotonin-dopamine activity modulator (SDAM), it acts as a novel partial agonist for D2 dopamine and serotonin 1A receptors while effectively blocking the serotonin 2A receptors, as well as noradrenergic alpha 1B and 2C receptors.
References
https://en.wikipedia.org/wiki/Brexpiprazole
https://www.drugbank.ca/drugs/DB09128
Maeda, K, et al. "Brexpiprazole I: in vitro and in vivo characterization of a novel serotonin-dopamine activity modulator." Journal of Pharmacology & Experimental Therapeutics 350.3(2014):589-604.
Maeda, K, et al. "Brexpiprazole II: antipsychotic-like and procognitive effects of a novel serotonin-dopamine activity modulator." Journal of Pharmacology & Experimental Therapeutics 350.3(2014):605.
Yoshimi, N, et al. "Effects of brexpiprazole, a novel serotonin-dopamine activity modulator, on phencyclidine-induced cognitive deficits in mice: a role for serotonin 5-HT1A receptors. " Pharmacology Biochemistry & Behavior 124(2014):245.
Properties of Brexpiprazole
| Melting point: | 179 - 181oC |
| Boiling point: | 675.2±55.0 °C(Predicted) |
| Density | 1.245±0.06 g/cm3(Predicted) |
| storage temp. | Refrigerator |
| solubility | Dichloromethane (Slightly), Chloroform (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 11.22±0.70(Predicted) |
| color | White to Off-White |
| InChI | InChI=1S/C25H27N3O2S/c29-25-9-7-19-6-8-20(18-22(19)26-25)30-16-2-1-11-27-12-14-28(15-13-27)23-4-3-5-24-21(23)10-17-31-24/h3-10,17-18H,1-2,11-16H2,(H,26,29) |
Safety information for Brexpiprazole
Computed Descriptors for Brexpiprazole
| InChIKey | ZKIAIYBUSXZPLP-UHFFFAOYSA-N |
| SMILES | N1C2=C(C=CC(OCCCCN3CCN(C4=C5C=CSC5=CC=C4)CC3)=C2)C=CC1=O |
Brexpiprazole manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 Brexpiprazole 98%View Details
Brexpiprazole 98%View Details -
 Brexpiprazole 98%View Details
Brexpiprazole 98%View Details -
 913611-97-9 Brexpiprazole 98%View Details
913611-97-9 Brexpiprazole 98%View Details
913611-97-9 -
 913611-97-9 Brexpiprazole 99%View Details
913611-97-9 Brexpiprazole 99%View Details
913611-97-9 -
 913611-97-9 98%View Details
913611-97-9 98%View Details
913611-97-9 -
 Brexpiprazole 98%View Details
Brexpiprazole 98%View Details
913611-97-9 -
 Brexpiprazole 913611-97-9 99%View Details
Brexpiprazole 913611-97-9 99%View Details
913611-97-9 -
 Brexpiprazole 913611-97-9 99%View Details
Brexpiprazole 913611-97-9 99%View Details
913611-97-9
