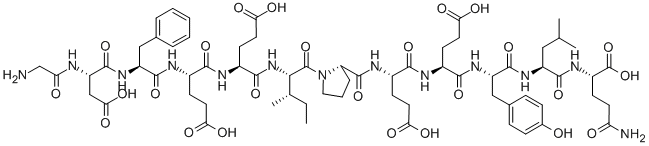
BNP-32 (HUMAN)
Synonym(s):BNP-32
- CAS NO.:124584-08-3
- Empirical Formula: C143H244N50O42S4
- Molecular Weight: 3464.05
- MDL number: MFCD00133149
- EINECS: 1312995-182-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-31 13:32:20
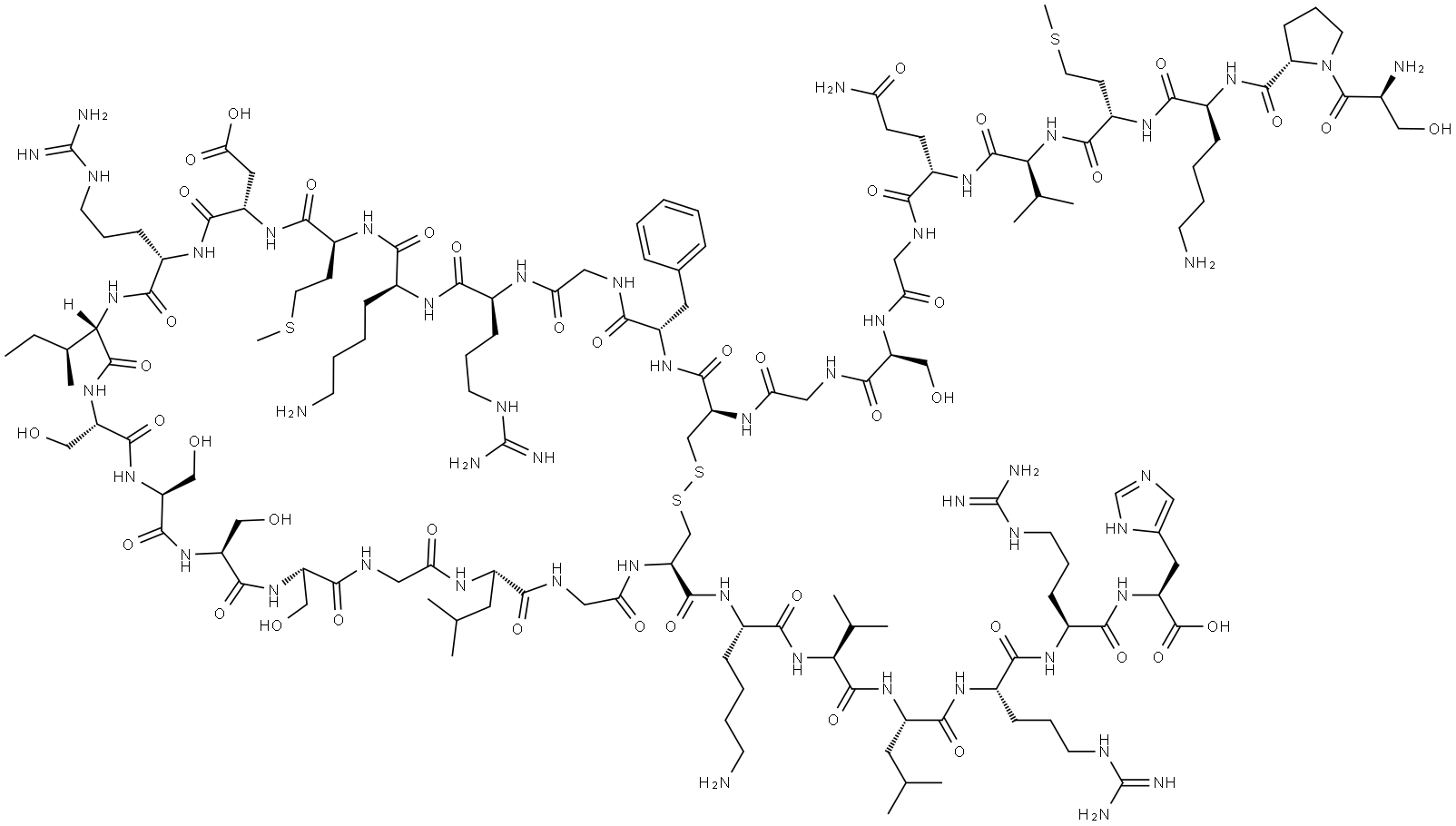
What is BNP-32 (HUMAN)?
Description
Nesiritide was introduced in the US as a new intravenous treatment for patients with acutely decompensated congestive heart failure who have dyspnea at rest or with minimal activity. Nesiritide is the first human recombinant form of the potent vasodilatory B-type natriuretic peptide (also known as brain natriuretic peptide), a naturally occurring 32 amino acid peptide with a disulfide-bonded 17 amino acid ring structure. Nesiritide binds to the Atype natriuretic peptide receptor on the surface of endothelial and smooth muscle cells stimulating production of the second messenger cGMP which mediates predominantly vascular smooth muscle cell relaxation. It also facilitates the elimination of sodium and water by the kidney and decreases the secretion of certain hormones, such as adrenalin, angiotensin II, aldosterone and endothelin, which provoke long-term detrimental effects including blood vessel constriction and blood pressure elevation. In clinical trials with heart failure patients, nesidtide dose-dependently reduced pulmonary capillary wedge pressure, right atrial pressure and systemic vascular resistance and increased cardiac index without affecting heart rate. The major adverse effect observed was dose-dependent hypotension. In a phase III comparative trial, nesiritide was found to be superior to iv. nitroglycerine in its haemodynamic effects as well as easier to administer and better tolerated. Nesiritide is cleared by proteolytic cleavage by the enzyme neutral endopeptidase NEP24.11 and by binding to the C-type natriuretic peptide receptor followed by endocytosis and intracellular lysosomal hydrolysis. Since it has a short half-life (18 min), nesiritide is administered as a 21μg/kg bolus infusion followed by a continuous maintenance infusion at 0.011μg/kg/min usually over 24-48 hours.
Originator
Scios (US)
The Uses of BNP-32 (HUMAN)
Treatment of congestive heart failure (renin-angiotensin system antagonist).
The Uses of BNP-32 (HUMAN)
Brain natriuretic peptide is originally isolated from brain, but is mainly produced in myoendocrine cells of the heart ventricles from which it is released into the circulation. It is involved in blood pressure control and cardiovascular homeostasis.
brand name
Natrecor (Biochemie, Austria).
General Description
The gene encoding brain natriuretic peptide-32 (BNP-32) is localized on human chromosome 1p36.22. It is implicated in several biological functions such as diuresis, natriuresis, hypotensive action and inhibition of aldosterone secretion. BNP-32 acts as a potential biomarker of high left ventricular-diastolic pressure in patients with symptomatic left ventricular (LV) dysfunction. Elevated expression of this protein is observed in acute myocardial infarction (AMI) patients.
Biochem/physiol Actions
Brain natriuretic peptide (type B natriuretic peptide) was originally isolated from brain, but is mainly produced in myoendocrine cells of the heart ventricles from which it is released into the circulation. It is involved in blood pressure control and cardiovascular homeostasis.
storage
Store at -20°C
Properties of BNP-32 (HUMAN)
| Density | 1.52±0.1 g/cm3(Predicted) |
| RTECS | EE1534000 |
| storage temp. | −20°C |
| form | powder |
| color | White to off-white |
| Water Solubility | Soluble in water at 1mg/ml |
| CAS DataBase Reference | 124584-08-3 |
Safety information for BNP-32 (HUMAN)
Computed Descriptors for BNP-32 (HUMAN)
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid Fmoc-Val-Cit-PAB DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonateRelated products of tetrahydrofuran
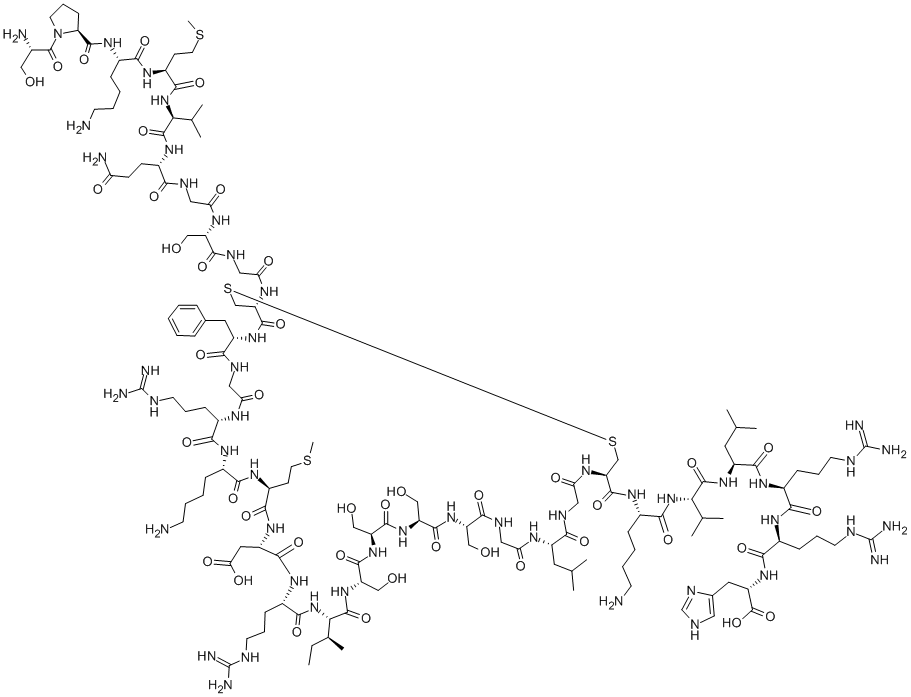



You may like
-
 Brain Natriuretic Peptide-32 human CAS 124584-08-3View Details
Brain Natriuretic Peptide-32 human CAS 124584-08-3View Details
124584-08-3 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8