Bitertanol
Synonym(s):3,3-Dimethyl-1-(4-phenylphenoxy)-1-(1,2,4-triazol-1-yl)butan-2-ol
- CAS NO.:55179-31-2
- Empirical Formula: C20H23N3O2
- Molecular Weight: 337.42
- MDL number: MFCD00055508
- EINECS: 259-513-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
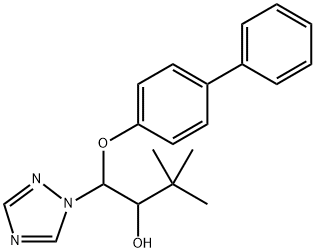
What is Bitertanol?
Description
Bitertanol is a broad-spectrum triazole fungicide that is active against a variety of fungi, including V. inaequalis and V. pirina, which are responsible for apple and pear scab, respectively, as well as S. mors-uvae and P. ribis, which are responsible for American gooseberry mildew and leaf spot in black currants, respectively. It is active against isolates of V. inaequalis (MICs = 0.6 and 1 μg/ml) but repeated use leads to resistance and cross-resistance (MICs = 9.8-13 μg/ml for bitertanol-resistant isolates). Bitertanol inhibits the cytochrome P450 (CYP450) isoform CYP3A4 (IC50 = 2.74 μM) and inhibits androgenic activity induced by the androgen receptor agonist DHT in a yeast two-hybrid assay (IC50 = 79.85 μM). In rats, bitertanol (10-300 mg/kg, i.p.) increases operant responding on one- and five-minute fixed interval schedules with no effect on motor activity.
Chemical properties
White Solid
The Uses of Bitertanol
Bitertanol is a foliar-applied triazole fungicide used to protect tropical and subtropical crops in agriculture, in particular bananas. Bitertanol has effects on behavior that are similar to those of psychomotor stimulants.
The Uses of Bitertanol
Agricultural fungicide.
The Uses of Bitertanol
Bitertanol is used as a seed treatment on winter wheat and barley and as a foliar treatment to control diseases on bananas, ornamentals, fruit and vegetables.
What are the applications of Application
Bitertanol is a triazole fungicide used to kill tropical and subtropical fungi in vitro
Definition
ChEBI: 1-(biphenyl-4-yloxy)-3,3-dimethyl-1-(1,2,4-triazol-1-yl)butan-2-ol is a member of the class of triazoles that is 3,3-dimethyl-1-(1,2,4-triazol-1-yl)butane-1,2-diol substituted at position O-1 by a biphenyl-4-yl group. It is a member of biphenyls, an aromatic ether, a member of triazoles and a secondary alcohol.
Agricultural Uses
Fungicide: A fungicide used to control a variety of diseases. Used as control for prunes when drying. Not currently registered in the U.S. or EU countries (pending). Used in many European, South American, Far Eastern and African countries, a well as in Australia for use on beans (all types) and various ornamentals. More than 20 global suppliers.
Trade name
BAYCOR®; BAY KWG 0599®; BAYMATSPRAY ®; BILOXAZOL®; KWG 0599®; SIBUTOL®, (with Fuberidazole)
Metabolic pathway
Bitertanol is a mixture of two diastereoisomeric pairs and this complicates its metabolism, each biotransfonnation affording a mixture of isomers. Metabolism has been studied under environmental conditions and in plants and mammals. Degradation in soil and sediments is slow and only small quantities of products (other than CO2 and bound residues) are produced. Most information is available for mammals in which metabolism occurs mainly by oxidative routes followed by conjugation. The information presented below is derived from the UK MAFF Pesticide Safety Directorate (PSD, 1994).
Degradation
The hydrolytic stability of [14C]bitertanowl as measured at pH 4, 7 and 9
over 30 days at 25 and 40 °C. No decomposition was observed and the
DT50 was estimated at >1 year at 25 °C.
Aqueous solutions of [14C-triazole]-and [14C-biphenyl]-bitertanol were
irradiated for 48 and 72 hours, respectively, using a mercury arc lamp
under conditions somewhat exceeding natural sunlight. Calculated DT50
values were 38-52 hours and an estimated environmental DT50 was considered
to be 11 days. 4-Hydroxybiphenyl (4) and 1,2,4-triazole (5) were
identified as major photolysis products accounting for 12% and 53% of
the radioactivity, respectively. These are shown in Scheme 1. At least six
minor products were detected but these were not identified.
Very little degradation occurred under soil surface photolysis conditions
simulating natural sunlight. No discrete products were detected.
Bound residue increased to only 5% of the total over 35 days.
Properties of Bitertanol
| Melting point: | 49-50°C |
| Boiling point: | 350°C |
| Density | 1.1676 (rough estimate) |
| vapor pressure | A 2.2 x l0-10 (20 °C)
B 2.5 x l0-9 (20 °C) |
| refractive index | 1.6200 (estimate) |
| Flash point: | 100 °C |
| solubility | Chloroform: slightl soluble,DMSO: Slightly Soluble,Methanol: Slightly Soluble |
| form | neat |
| Water Solubility | A 2.7 mg l-1 (20 °C)
B 1.1 mg l-1 (20 °C) |
| pka | 13.40±0.20(Predicted) |
| Merck | 13,1301 |
| BRN | 620948 |
| NIST Chemistry Reference | 1H-1,2,4-triazole-1-ethanol, «beta»-([1,1'-biphenyl]-4-yloxy)-«alpha»-(1,1-dimethylethyl)-(55179-31-2) |
| EPA Substance Registry System | Bitertanol (55179-31-2) |
Safety information for Bitertanol
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P501:Dispose of contents/container to..… |
Computed Descriptors for Bitertanol
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1