BISMUTH OXYCHLORIDE
Synonym(s):Bismuth(III) chloride oxide;Bismuthyl(III) chloride;Chlorobismuth oxide
- CAS NO.:7787-59-9
- Empirical Formula: BiClO
- Molecular Weight: 260.43
- MDL number: MFCD00010895
- EINECS: 232-122-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
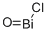
What is BISMUTH OXYCHLORIDE?
Chemical properties
Bismuth oxychloride is a white Lustorus powder, It is an inorganic white pigment commonly used in foundations, blushes and other color cosmetics. Bismuth oxychloride usually comes in two forms: diamond and pearl finishes. The diamond is shimmery and the pearl is more matte.
Bismuth is the least toxic of its periodic table neighbors like lead, tin, antimony and polonium. Bismuth in itself is not safe for use in cosmetics, and must be refined and combined with other elements to produce bismuth oxychloride.

Bismuth occurs naturally, but in very small amounts. Most of the bismuth produced in the USA is as a by-product from refining lead, tin, copper, silver and gold ores.
Once the bismuth has been harvested, it is further refined through several processes to remove dangerous elements like lead. Then it is chlorinated, which gives us bismuth chloride (BiCl3). It still poses a severe risk at this point, smelling of hydrochloric acid. When bismuth chloride is combined with water, it starts to decompose and part of the chlorine is replaced by oxygen from the water. This process is called hydrolysis. The remaining compound is bismuth, chlorine and oxygen = Bismuth oxychloride.
Physical properties
White powder or tetragonal crystals; density 7.72 g/cm3; practically insoluble in water, alcohol and acetone; soluble in hydrochloric and nitric acids (with decomposition); Ksp 7.0 x 10-9.The layered structure gives rise to the pearlescent properties of this material.
The Uses of BISMUTH OXYCHLORIDE
bismuth oxychloride is an inorganic color additive. It is used mostly in makeup manufacturing and rarely in skin care formulations. It may also be used for pearlization in cosmetics.
The Uses of BISMUTH OXYCHLORIDE
In face powders; as pigment; manufacture of artificial pearls, dry-cell cathodes.
The Uses of BISMUTH OXYCHLORIDE
It is used as skin protective's, thickeners, and absorbent agents. It act as a coloring agents in cosmetics.
Preparation
Bismuth oxychloride is made by treating bismuth chloride with water and then drying the white precipitate so formed to expel a molecule of water:
BiCl3 + 2H2O → Bi(OH)2Cl + 2HCl
Bi(OH)2Cl → BiOCl + H2O
Also, the compound is prepared by treating a dilute nitric acid solution of bismuth nitrate with sodium chloride.
General Description
Odorless white solid. Sinks in water.
Reactivity Profile
BISMUTH OXYCHLORIDE emits toxic fumes of chloride ion and bismuth when heated to decomposition [USCG, 1999].
Health Hazard
Contact with dust causes mild eye irritation and can cause skin rashes.
Flammability and Explosibility
Not classified
Structure
The structure of bismuth oxychloride can be thought of as consisting of layers of Cl?, Bi3+ and O2? ions. These ions are ordered as Cl–Bi–O–Bi–Cl–Cl–Bi–O–Bi–Cl, i.e., with alternating anions (Cl?, O2?) and cations (Bi3+).
Properties of BISMUTH OXYCHLORIDE
| Melting point: | melts at low red heat [MER06] |
| Density | 7.72 g/mL at 25 °C (lit.) |
| refractive index | 2.15 |
| solubility | H2O: insoluble(lit.) |
| form | Powder |
| Specific Gravity | 7.72 |
| color | Very pale brown |
| Odor | at 100.00?%. odorless |
| Water Solubility | Soluble in HCl, HNO3. Insoluble in water, alcohol. |
| Merck | 14,1263 |
| Solubility Product Constant (Ksp) | pKsp: 30.75 |
| CAS DataBase Reference | 7787-59-9(CAS DataBase Reference) |
| EPA Substance Registry System | Bismuthine, chlorooxo- (7787-59-9) |
Safety information for BISMUTH OXYCHLORIDE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for BISMUTH OXYCHLORIDE
| InChIKey | GLQBXSIPUULYOG-UHFFFAOYSA-M |
BISMUTH OXYCHLORIDE manufacturer
Dhara Industries
Sajan Overseas Pvt Ltd
Rivashaa Agrotech Biopharma Pvt. Ltd.
DeFINE CHEMICALS
Samuh Laxmi Chemicals (Bom) P Ltd
Monopoly Innovations Pvt. Ltd.
ARRAKIS INDUSTRIES LLP
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 BISMUTH OXY CHLORIDE 99%View Details
BISMUTH OXY CHLORIDE 99%View Details -
 Bismuth(III) oxychloride 99%View Details
Bismuth(III) oxychloride 99%View Details -
 Bismuth(III) chloride oxide CAS 7787-59-9View Details
Bismuth(III) chloride oxide CAS 7787-59-9View Details
7787-59-9 -
 Bismuth(III) chloride oxide CAS 7787-59-9View Details
Bismuth(III) chloride oxide CAS 7787-59-9View Details
7787-59-9 -
 Bismuth(III) chloride oxide CAS 7787-59-9View Details
Bismuth(III) chloride oxide CAS 7787-59-9View Details
7787-59-9 -
 Bismuth(III) chloride oxide CAS 7787-59-9View Details
Bismuth(III) chloride oxide CAS 7787-59-9View Details
7787-59-9 -
 Bismuth(III) chloride oxide CAS 7787-59-9View Details
Bismuth(III) chloride oxide CAS 7787-59-9View Details
7787-59-9 -
 Bismuth Oxychloride CASView Details
Bismuth Oxychloride CASView Details