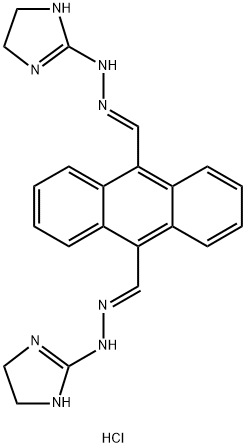
Bisantrene
- CAS NO.:78186-34-2
- Empirical Formula: C22H22N8
- Molecular Weight: 398.46
- MDL number: MFCD00872137
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-17 16:00:36
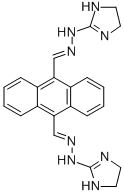
What is Bisantrene?
Originator
Bisantrene hydrochloride,ZYF Pharm Chemical
The Uses of Bisantrene
Antineoplastic.
Definition
ChEBI: A hydrazone resulting from the formal condensation of both of the aldehyde groups of anthracene-9,10-dicarbaldehyde with 2-hydrazinyl-4,5-dihydro-1H-imidazole.
Manufacturing Process
A 33.0 g (0.135 mole) of 2-methylthio-2-imidazoline hydroiodide is dissolved
in 300 ml of water and treated with 8 ml (0.16 mole) of hydrazine hydrate.
The mixture is stirred at room temperature for 20 hours and then taken to
dryness under reduced pressure. The residue is dissolved in 250 ml of water
and again taken to dryness under reduced pressure. The residue is
redissolved in 250 ml of water and added to a mixture of 250 ml of water, 25
ml of concentrated hydrochloric acid and 25 g of silver oxide. The resulting
mixture is stirred on a steam bath for 4 hours and then filtered. The filtrate is
reduced to dryness under reduced pressure. The residue is dissolved in 300
ml of ethanol and 20 ml of water at the boil, clarified and cooled at -10°C.
The precipitate is collected, washed with ethanol and ether and dried at 60°C
and then 110°C under reduced pressure. Yield of the 2-hydrazino-2-
imidazoline hydrochloride 11.6 g, melting point 177-180°C.
The 2-hydrazino-2-imidazoline monohydrochloride is converted to the
dihydrochloride by treatment with ethanol and concentrated hydrochloric acid.
A suspension of 3.46 g of the 2-hydrazino-2-imidazoline dihydrochloride and
2.34 g of 9,10-anthracenedicarboxaldehyde in 100 ml of ethanol is stirred and
heated under reflux for two hours. The mixture is cooled and the solid is
collected and washed with ethanol giving the desired product as a crystalline
orange solid, m.p. 288-289°C (dec.).
Therapeutic Function
Antineoplastic
Properties of Bisantrene
| Boiling point: | 646.3±65.0 °C(Predicted) |
| Density | 1.41±0.1 g/cm3(Predicted) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | deionized water: soluble8mg/mL |
| form | solid |
| pka | 9.78±0.10(Predicted) |
| color | Orange to red |
Safety information for Bisantrene
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H400:Hazardous to the aquatic environment, acute hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. |
Computed Descriptors for Bisantrene
| InChIKey | NJSMWLQOCQIOPE-OCHFTUDZSA-N |
| SMILES | C1(/C=N/NC2=NCCN2)=C2C=CC=CC2=C(/C=N/NC2=NCCN2)C2C=CC=CC1=2 |
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3,4-Dibenzyloxybenzaldehyde 4-Hydrazinobenzoic acid 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 3-NITRO-2-METHYL ANILINE 4-IODO BENZOIC ACID 4-HYDROXY BENZYL ALCOHOL 4-(3-chloropropyl)morpholine phenylhydrazine hydrochloride (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 4-methoxy-3,5-dinitropyridine 2-(Cyanocyclohexyl)acetic acid 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamateRelated products of tetrahydrofuran

You may like
-
 Bisantrene dihydrochloride CAS 78186-34-2View Details
Bisantrene dihydrochloride CAS 78186-34-2View Details
78186-34-2 -
 (9H-fluoren-9-yl)methyl (2,5-dioxopyrrolidin-1-yl) carbonate 82911-69-1 98.0%View Details
(9H-fluoren-9-yl)methyl (2,5-dioxopyrrolidin-1-yl) carbonate 82911-69-1 98.0%View Details
82911-69-1 -
 13057-17-5 95.0%View Details
13057-17-5 95.0%View Details
13057-17-5 -
 4-bromoaniline 106-40-1 99.0%View Details
4-bromoaniline 106-40-1 99.0%View Details
106-40-1 -
 1421517-99-8 99.0%View Details
1421517-99-8 99.0%View Details
1421517-99-8 -
 5-bromo-2-chlorobenzoic acid 99.0%View Details
5-bromo-2-chlorobenzoic acid 99.0%View Details
21739-92-4 -
 2-methyl-5-nitrophenol 98.0%View Details
2-methyl-5-nitrophenol 98.0%View Details
5428-54-6 -
 15761-38-3 97.0%View Details
15761-38-3 97.0%View Details
15761-38-3