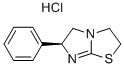
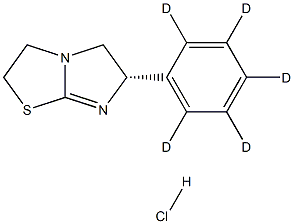
Levamisole hydrochloride
Synonym(s):(−)-Tetramisole hydrochloride;(S)-(−)-6-Phenyl-2,3,5,6-tetrahydroimidazo[2,1-b]thiazole hydrochloride;L (−)-2,3,5,6-Tetrahydro-6-phenylimidazo[2,1-b]thiazole hydrochloride;Levamisole hydrochloride
- CAS NO.:16595-80-5
- Empirical Formula: C11H13ClN2S
- Molecular Weight: 240.75
- MDL number: MFCD00012675
- EINECS: 240-654-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:57
What is Levamisole hydrochloride?
Chemical properties
White to light yellow crystalline powder, odorless, bitter taste. Melting point 227-229℃. Soluble in water, methanol, ethanol and glycerol, slightly soluble in chloroform and aether; insoluble in acetone. Stable under acidic conditions, but easy to decompose and fail under alkaline conditions.
Originator
Solaskil,Specia,France,1971
The Uses of Levamisole hydrochloride
Levamisole hydrochloride is an anthelmintic. The activity of levamisole is about twice as high as that of abscisicidal, and the toxicity and side effects are lower. Levamisole can cause muscle paralysis of roundworms and then excrete them in the feces. It is mainly used as an anti-worm and anti-hookworm.
The Uses of Levamisole hydrochloride
Biological response modifier with anthelmintic activity. Anthelmintic (nematodes); immunomodulator.
What are the applications of Application
Levamisole Hydrochloride is an immunomodulatory agent in human micro-and macro vascular endothelial cells
Preparation
Levamisole hydrochloride was prepared from racemic tetraimidazole by splitting, alkali analysis and acidification into salt.
Dibenzoyl-D-tartaric acid is boiled in water for 40min, hydrolyzed and neutralized with sodium hydroxide solution to pH 7.5, and then added to racemic tetraimidazole. The levamisole is precipitated by generating salt with bisbenzoyl-tartaric acid. After separation, add sodium hydroxide solution to PH=9 and decompose levamisole. Then dissolved in dilute hydrochloric acid solution, after decolorization by activated carbon, the filtrate was concentrated nearly dry, and crystallized by adding acetone and cooling to 0℃. Filtered and dried to obtain levamisole hydrochloride.
Definition
ChEBI: Levamisole hydrochloride is an organic molecular entity.
brand name
Ergamisol (Janssen);Vermisol.
Therapeutic Function
Antiinflammatory
Biochem/physiol Actions
Shows both immunostimulant and immunosuppressant effects, depending on several controllable factors. Very effective in treatment of ascariasis (hookworm infestation). Useful in chemotherapy of colorectal cancers, possibly due to its stimulation of IL-1 production and direct activation of macrophages.
Pharmacology
Levamisole Hydrochloride is the hydrochloride salt of the synthetic imidazothiazole derivative levamisole with anthelminthic and immunomodulating activities. In immunosuppressed states, levamisole may restore immune function by: 1) stimulating antibody formation, 2) stimulating T-cell activation and proliferation, 3) potentiating monocyte and macrophage phagocytosis and chemotaxis and 4) increasing neutrophil mobility, adherence, and chemotaxis.
Pharmacokinetics
Levamisole is rapidly absorbed from all routes of administration, with peak blood levels occurring within an hour followed by rapid metabolism and depletion principally via urinary excretion, with an elimination half-life of approximately 4 h.
Mode of action
Levamisole is a cholinergic receptor agonist and elicits spastic muscle paralysis due to prolonged activation of the excitatory nicotinic acetylcholine receptors (nAChR) on nematode body wall muscle.
Toxicity evaluation
Symptoms of levamisole toxicity mimic organophosphate toxicity (salivation, lacrimation, urination and defecation, hyperesthesia, seizures and irritability). There is no antidote for levamisole toxicity.
The World Health Organization reviewed hematological studies in animals and humans and derived acceptable daily intake for levamisole as 0.006 mg/kg body weight. This suggests a person can ingest 0.36 mg of levamisole/day over a lifetime without any appreciable risk.
levamisole
Properties of Levamisole hydrochloride
| Melting point: | 266-267 °C(lit.) |
| alpha | -128 º (c=5, H2O) |
| refractive index | -126 ° (C=1, H2O) |
| Flash point: | 9℃ |
| storage temp. | 2-8°C |
| solubility | Freely soluble in water, soluble in ethanol (96 per cent), slightly soluble in methylene chloride. |
| form | Crystalline Powder |
| color | White to almost white |
| Water Solubility | 210 g/L (20 ºC) |
| Merck | 14,5459 |
| BRN | 4358988 |
| Stability: | Hygroscopic |
| InChI | InChI=1/C11H12N2S.ClH/c1-2-4-9(5-3-1)10-8-13-6-7-14-11(13)12-10;/h1-5,10H,6-8H2;1H/t10-;/s3 |
| CAS DataBase Reference | 16595-80-5(CAS DataBase Reference) |
| EPA Substance Registry System | Levamisole hydrochloride (16595-80-5) |
Safety information for Levamisole hydrochloride
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral |
Computed Descriptors for Levamisole hydrochloride
| InChIKey | LAZPBGZRMVRFKY-HNCPQSOCSA-N |
| SMILES | [C@H]1(N=C2SCCN2C1)C1=CC=CC=C1.Cl |&1:0,r| |
Levamisole hydrochloride manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 Levamisole HCL 99%View Details
Levamisole HCL 99%View Details -
 16595-80-5 99%View Details
16595-80-5 99%View Details
16595-80-5 -
 Levamisole HCl 98% CAS 16595-80-5View Details
Levamisole HCl 98% CAS 16595-80-5View Details
16595-80-5 -
 Levamisole Hydrochloride CAS 16595-80-5View Details
Levamisole Hydrochloride CAS 16595-80-5View Details
16595-80-5 -
![Levamisole Hydrochloride [for Biochemical Research] CAS 16595-80-5](https://img.chemicalbook.in//Content/image/CP5.jpg) Levamisole Hydrochloride [for Biochemical Research] CAS 16595-80-5View Details
Levamisole Hydrochloride [for Biochemical Research] CAS 16595-80-5View Details
16595-80-5 -
 Levamisole hydrochloride CAS 16595-80-5View Details
Levamisole hydrochloride CAS 16595-80-5View Details
16595-80-5 -
 Levamisole hydrochloride CAS 16595-80-5View Details
Levamisole hydrochloride CAS 16595-80-5View Details
16595-80-5 -
 Levamisole hydrochloride CAS 16595-80-5View Details
Levamisole hydrochloride CAS 16595-80-5View Details
16595-80-5