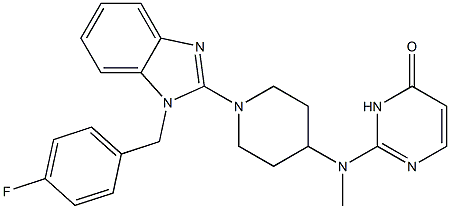
Mizolastine
- CAS NO.:108612-45-9
- Empirical Formula: C24H25FN6O
- Molecular Weight: 432.49
- MDL number: MFCD23140913
- EINECS: 1308068-626-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 20:33:22
What is Mizolastine?
Description
Mizolastine was marketed in Germany and Switzerland as Mizollen for the symptomatic relief of seasonal and perennial allergic rhinoconjunctivitis and urticaria. Mizolastine is a new long-acting, orally active antihistaminic agent with a rapid onset of action ; the two most recent H1 antagonists launched were fexofenadine, metabolite of terfenadine (Sepracor, 1996) and Olopatadine (Kyowa Hakko, 1997). Mizolastine can be prepared in 2 steps from 2-chloro 1- (4-fluorobenzyl)benzimidazole by successive condensations of appropriate amine and thioether. Mizolastine selectively blocks the peripheral H1 receptors (but not the serotonergic, noradrenergic, muscarinic receptors) with a minimal occupancy of brain receptors, and therefore does not elicit any sedative effects. Moreover, Mizolastine does not produce cardiac rhythm disorders which have been associated with certain non-sedating antihistamines in humans.
Chemical properties
White Solid
Originator
Synthelabo (France)
The Uses of Mizolastine
A highly selective histamine H1-receptor antagonist (with no anticholinergic, antiadrenergic, or antiserotonin activity) for use in the treatment of allergic disorders, especially rhinitis and urticaria.
The Uses of Mizolastine
Allergic rhinitis;Blocking H1 receptors
Definition
ChEBI: Mizolastine is a member of benzimidazoles.
Background
Mizolastine is under investigation in clinical trial NCT01928316 (A Bioequivalence Study of Domestic (Made in China) and Imported Mizolastine Tablets in Healthy Volunteers).
brand name
Mizollen
Clinical Use
Antihistamine:
Symptomatic relief of allergy, e.g. hayfever, urticaria
Drug interactions
Potentially hazardous interactions with other drugs
Anti-arrhythmics: increased risk of ventricular
arrhythmias - avoid with amiodarone, disopyramide,
flecainide, mexiletine, procainamide and
propafenone.
Antibacterials: metabolism possibly inhibited by
macrolides - avoid; increased risk of ventricular
arrhythmias with moxifloxacin - avoid.
Antidepressants: risk of ventricular arrhythmias with
citalopram and escitalopram - avoid.
Antifungals: metabolism inhibited by itraconazole
and ketoconazole and possibly imidazoles - avoid.
Antimalarials: avoid with piperaquine with
artenimol.
Antivirals: concentration possibly increased by
ritonavir; increased risk of ventricular arrhythmias
with saquinavir - avoid.
Beta-blockers: increased risk of ventricular
arrhythmias with sotalol - avoid.
Ciclosporin: use with caution due to inhibition of
ciclosporin metabolism.
Cytotoxics: possible increased risk of ventricular
arrhythmias with vandetanib.
Avoid concomitant treatment with any drug that
could prolong QT interval.
Caution with drugs that inhibit cytochrome P450
enzymes (may elevate mizolastine levels)
Metabolism
Mainly metabolised by glucuronidation although other metabolic pathways are involved, including metabolism by the cytochrome P450 isoenzyme CYP3A4, with the formation of inactive hydroxylated metabolites
Metabolism
Not Available
Properties of Mizolastine
| Melting point: | 217° |
| Density | 1.34±0.1 g/cm3(Predicted) |
| storage temp. | Refrigerator |
| solubility | Chloroform (Slightly, Heated), DMSO, Methanol (Slightly, Heated) |
| form | Solid |
| pka | 9.73±0.40(Predicted) |
| color | White |
| Merck | 14,6221 |
Safety information for Mizolastine
Computed Descriptors for Mizolastine
Mizolastine manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 108612-45-9 Mizolastine 98%View Details
108612-45-9 Mizolastine 98%View Details
108612-45-9 -
 108612-45-9 98%View Details
108612-45-9 98%View Details
108612-45-9 -
 Mizolastine 98%View Details
Mizolastine 98%View Details
108612-45-9 -
 Mizolastine CAS 108612-45-9View Details
Mizolastine CAS 108612-45-9View Details
108612-45-9 -
 Mizolastine 98% CAS 108612-45-9View Details
Mizolastine 98% CAS 108612-45-9View Details
108612-45-9 -
 Mizolastine CAS 108612-45-9View Details
Mizolastine CAS 108612-45-9View Details
108612-45-9 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1