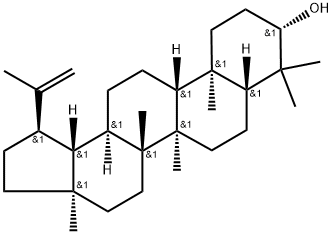
Betulin
Synonym(s):Trochol;Betulinol;Betulol;Lup-20(29)-ene-3β,28-diol;Lup-20(29)-ene-3β,28-diol, Sterol Regulatory Element Binding Protein Processing Inhibitor, Betulin
- CAS NO.:473-98-3
- Empirical Formula: C30H50O2
- Molecular Weight: 442.72
- MDL number: MFCD00016802
- EINECS: 207-475-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-25 11:31:46
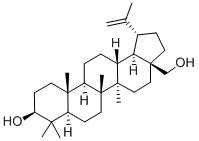
What is Betulin?
Description
Sterol regulatory element binding protein 2 (SREBP-
Chemical properties
crystals
The Uses of Betulin
Betulin may be used in the preparation of betulinic acid, which shows anti-HIV, antimalarial and anti-inflammatory activities.
The Uses of Betulin
antineoplastic, antihyperlipidemia
The Uses of Betulin
birch bark extract is described as having anti-irritant and antiseptic properties, and effective in acne treatment. It is used to make sunburn products, soothing lotions, and aftershaves. The oil is astringent and is mainly used for its curative effects, especially in cases of acne and eczema. In folkloric medicine, birch bark extract was considered good for bathing skin eruptions. Destructive distillation of the bark’s white epidermis yields an empyreumatic oil known as oil of birch tar, Oleum rusci, or dagget. This is a thick, bituminous, brownish-black liquid with a pungent, balsamic odor. It contains a high percentage of methylsalicilate, creosol, and guaiacol and is almost identical to wintergreen oil.
What are the applications of Application
Betulin is a triterpene with a pentacyclic ring structure
Definition
ChEBI: A pentacyclic triterpenoid that is lupane having a double bond at position 20(29) as well as 3beta-hydroxy and 28-hydroxymethyl substituents.
General Description
A cell-permeable pentacyclic triterpenoid from birch bark extract that interacts with SCAP (SREBP cleavage activating protein) and prevents SREBP (sterol regulatory element-binding protein) Golgi proteolytic activation (1 to 13.55 μM in Rat hepatocytes CRL-1601) via a similar mechanism as oxysterols by inducing the association of SCAP with Insig-1 (insulin-induced gene-1), thereby causing SCAP-SREBPs complex ER retention. Unlike oxysterols, Betulin does not activate LXR to induce concomitant HMGCR (HMG-CoA reductase) degradation and SREBP-1 up-regulation. While both Betulin and HMGCR inhibitor Lovastatin (Cat. No. 438185) inhibit cellular cholesterol synthesis (by ~70% in CRL-1601 cultures with respective compound at 13.55 and 1 μM concentration), only Betulin suppresses cellular fatty acid synthesis (by ~55% at 13.55 μM in CRL-1601). Betulin is shown to exhibit comparable in vivo efficacy as Lovastatin (both dosed at 30 mg/kg/day with chow) in ameliorate high fat/cholesterol diet-induced obesity (% fat/lean tissue ratio increase from non-fat diet mice = 167, 44, and 33 in mice consuming fat diet alone, with Lovastatin, with Betulin, respectively). However, only Betulin is demonstrated to lower fasting blood glucose and insulin levels and reduce fat cell size in white adipose tissue in high fat diet mice. Fatostatin (Cat. No. 341329) in comparison does not target SCAP via sterol-binding site, nor does it induce SCAP binding to Insig-1.
Anticancer Research
In addition, triterpenoids (betulin and its derivatives) isolated from HSSEactivated the signaling pathway regulated by p53 family genes, leading to the inhibition of BC cell viability or even the induction of apoptosis. And also, theseresearchers found that all the triterpenoids had no effect on normal breast cells.These findings provide an important basis for the use of those triterpenoids in thedevelopment of alternative therapies for breast cancer treatment (Hsu et al. 2015).
Properties of Betulin
| Melting point: | 256-257 °C(lit.) |
| Boiling point: | 493.26°C (rough estimate) |
| alpha | D15 +20° (c = 2 in pyridine) |
| Density | 0.9882 (rough estimate) |
| refractive index | 1.5045 (estimate) |
| storage temp. | 2-8°C |
| solubility | Chloroform (Slightly), Methanol (Slightly, Heated) |
| form | Off-white powder |
| pka | 15.10±0.10(Predicted) |
| color | Pale Brown to Beige |
| Merck | 14,1189 |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 473-98-3(CAS DataBase Reference) |
| NIST Chemistry Reference | Betulin(473-98-3) |
Safety information for Betulin
| Signal word | Warning |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H371:Specific target organ toxicity, single exposure |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for Betulin
| InChIKey | FVWJYYTZTCVBKE-ROUWMTJPSA-N |
Betulin manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran


You may like
-
 Betulin >98%View Details
Betulin >98%View Details
473-98-3 -
 Betulin 98% (HPLC) CAS 473-98-3View Details
Betulin 98% (HPLC) CAS 473-98-3View Details
473-98-3 -
 Betulin, 90% CAS 473-98-3View Details
Betulin, 90% CAS 473-98-3View Details
473-98-3 -
 Betulinol CAS 473-98-3View Details
Betulinol CAS 473-98-3View Details
473-98-3 -
 Betulin, 70% CAS 473-98-3View Details
Betulin, 70% CAS 473-98-3View Details
473-98-3 -
 SREBP Processing Inhibitor, Betulin CAS 473-98-3View Details
SREBP Processing Inhibitor, Betulin CAS 473-98-3View Details
473-98-3 -
 Betulin CAS 473-98-3View Details
Betulin CAS 473-98-3View Details
473-98-3 -
 Betulin CAS 473-98-3View Details
Betulin CAS 473-98-3View Details
473-98-3
