Benzylidene-bis(tricyclohexylphosphine)dichlororuthenium
Synonym(s):Benzylidene-bis(tricyclohexylphosphine)dichlororuthenium;Bis(tricyclohexylphosphine)benzylidine ruthenium(IV) dichloride;Dichloro(benzylidene)bis(tricyclohexylphosphine)ruthenium(II);Grubbs Catalyst 1st Generation;Grubbs Catalyst M1a (C823)
- CAS NO.:172222-30-9
- Empirical Formula: C43H73Cl2P2Ru
- Molecular Weight: 823.96
- MDL number: MFCD01074452
- EINECS: 629-485-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
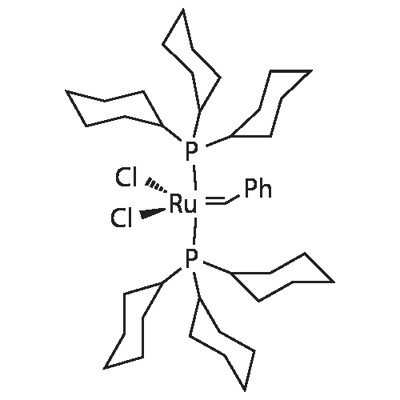
What is Benzylidene-bis(tricyclohexylphosphine)dichlororuthenium?
Description
Phenylmethylenebis(tricyclohexylphosphorus)ruthenium dichloride is a ruthenium catalyst. Ruthenium catalysts are highly resistant to polar monomers, enabling the polymerization of many polar monomers to prepare cyclic olefin copolymers. Cyclic olefin copolymers can be widely used in the manufacture of various optical, information, electrical, and medical materials.
Chemical properties
bright purple powder
The Uses of Benzylidene-bis(tricyclohexylphosphine)dichlororuthenium
Catalysis of olefin metathesis including ring-closing of dienes, cross metathesis and ring-opening methathesis polymerizations (ROMP).
The Uses of Benzylidene-bis(tricyclohexylphosphine)dichlororuthenium
First metathesis catalyst to be widely used in organic synthesis. Useful for acyclic diene metathesis polymerization (ADMET), Ring-Opening Metathesis Polymerization (ROMP) of strained cyclic olefins, ring opening metathesis (ROM), olefin cross metathesis (CM) and ring closing metathesis (RCM) of terminal olefins under a variety of reactions conditions.
The Uses of Benzylidene-bis(tricyclohexylphosphine)dichlororuthenium
suzuki reaction
What are the applications of Application
Phenylmethylenebis(tricyclohexylphosphorus)ruthenium dichloride can be widely used in organic synthesis, the first metathesis catalyst; under a wide range of reaction conditions, it is effective for the ring-opening metathesis of strained cyclic olefins of terminal olefins Polymerization, cross-metathesis and ring-closure metathesis units of olefins and vinyl alcohol decomposition of internal olefins.
Purification Methods
Wash it repeatedly with Me2CO and MeOH and dry it in a vacuum. Alternatively dissolve it in CH2Cl2, concentrate it to half its volume, filter, add MeOH to precipitate it as purple microcrystals. Filter these off, wash several times with Me2CO and MeOH and dry them in a vacuum for several hours. [Scwab et al. J Am Chem Soc 118 100 1996, Miller et al. J Am Chem Soc 118 9606 1996, Furstner & Langermann J Am Chem Soc 119 9130 1997.] § A polymer supported version is available [Schwab et al. Angew Chem (Intl Edn) 34 2039 1995.].
Properties of Benzylidene-bis(tricyclohexylphosphine)dichlororuthenium
| Melting point: | 153 °C (dec.)(lit.) |
| storage temp. | 2-8°C |
| solubility | Chloroform (Slightly), Dichloromethane (Sparingly) |
| color | Purple |
| Merck | 13,4565 |
| InChI | InChI=1S/2C18H33P.C7H5.2ClH.Ru/c2*1-4-10-16(11-5-1)19(17-12-6-2-7-13-17)18-14-8-3-9-15-18;1-7-5-3-2-4-6-7;;;/h2*16-18H,1-15H2;2-6H;2*1H;/q;;-2;;;+2 |
| CAS DataBase Reference | 172222-30-9 |
Safety information for Benzylidene-bis(tricyclohexylphosphine)dichlororuthenium
| Signal word | Warning |
| Pictogram(s) |
 Flame Flammables GHS02 |
| GHS Hazard Statements |
H228:Flammable solids |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. |
Computed Descriptors for Benzylidene-bis(tricyclohexylphosphine)dichlororuthenium
| InChIKey | PNPBGYBHLCEVMK-UHFFFAOYSA-L |
| SMILES | [Ru+4]([Cl-])([Cl-])(=[C-2]C1C=CC=CC=1)(P(C1CCCCC1)(C1CCCCC1)C1CCCCC1)P(C1CCCCC1)(C1CCCCC1)C1CCCCC1 |
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

![2-[[(2-ethylphenyl)(2-hydroxyethyl)amino]methyl]-3,3-difluoro-Propanenitrile](https://img.chemicalbook.in/CAS/GIF/2647-14-5.gif)

You may like
-
 Grubbs catalyst, 1st generation, 97% CAS 172222-30-9View Details
Grubbs catalyst, 1st generation, 97% CAS 172222-30-9View Details
172222-30-9 -
 Benzylidene-bis(tricyclohexylphosphine)dichlororuthenium Grubbs Catalyst 1st Gen CAS 172222-30-9View Details
Benzylidene-bis(tricyclohexylphosphine)dichlororuthenium Grubbs Catalyst 1st Gen CAS 172222-30-9View Details
172222-30-9 -
 Grubbs Catalyst® M102 CAS 172222-30-9View Details
Grubbs Catalyst® M102 CAS 172222-30-9View Details
172222-30-9 -
 Grubbs Catalyst® M102 ChemBeads CAS 172222-30-9View Details
Grubbs Catalyst® M102 ChemBeads CAS 172222-30-9View Details
172222-30-9 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4
