Ruthenium(III) chloride
- CAS NO.:10049-08-8
- Empirical Formula: RuCl3
- Molecular Weight: 207.43
- MDL number: MFCD00011208
- EINECS: 233-167-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
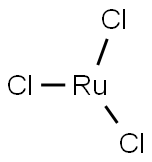
What is Ruthenium(III) chloride?
Chemical properties
The trihydrate, RuCl3·3H2O, is the usual commercial form. Aqueous solutions of the trihydrate are a straw color in dilute solution and red-brown in concentrated solution. Ruthenium (III) chloride in solution apparently forms a variety of aquo- and hydroxy complexes.
The Uses of Ruthenium(III) chloride
Ruthenium(III) trichloride is used for technical analysis in chemistry laboratories. It is highly toxic.
Ruthenium(III) chloride is a catalyst that is used in the synthesis of 2,3-Pyridinedicarboxylic Acid-13C3, 15N, where the unlabelled analog is an inhibitor of glucose synthesis.
Physical properties
Ruthenium(III) chloride is a reddish brown or black leafy crystal, easily deliquescent. It forms a dark red solution. Relative density 3.11, decomposed into monomers when it is higher than 500°C. It is insoluble in cold water and carbon disulfide. Insoluble in cold water and carbon disulfide, decompose in hot water, insoluble in ethanol, soluble in hydrochloric acid.
The Uses of Ruthenium(III) chloride
Ruthenium(III) chloride is widely used as a starting material of ruthenium complexes. It acts as a catalyst used in the oxidative cyclization of 1,7-dienes to oxepane diols. It is used in the hydroxylation of tertiary hydrocarbons in combination of periodate or bromate. It is involved as a catalyst in the synthesis of 2,3-pyridinedicarboxylic acid-13C3, 15N, where the unlabelled analog is an inhibitor of glucose synthesis.
What are the applications of Application
Ruthenium(III) chloride is a useful catalyst
Production Methods
RuCl3, is made by direct chlorination of the metal at 700 °C (1,292 °F). Two allotropic forms result. The trihydrate is made by evaporating an HCl solution of ruthenium (III) hydroxide to dryness or reducing ruthenium (VIII) oxide in a HCl solution.
What are the applications of Application
Ruthenium(III) trichloride is highly toxic and finds application in chemistry laboratories for technical analysis, particularly in the testing of sulfur trioxide. Ruthenium trichloride is by far the best starting material for the synthesis of compounds of the metal. Like osmium, ruthenium exhibits a wide range of oxidation states in its complexes (VIII to —II), and all of these may be reached from RuCl3 since, although it is stable, it can easily be oxidised or reduced. It is most commonly used in the hydrated form, this being soluble in many solvents, but for anhydrous or solid-state reactions β-RuCl3 is the best source.
Reactions
Ruthenium(III) chloride interacts with potassium iodide to produce an iodide precipitate, precipitates as ruthenium trisulphide when hydrogen sulphide is introduced into the solution, and can form the corresponding ammonia, cyanide, and nitrite complexes with ammonia, potassium cyanide, and potassium nitrite complexes, and is reduced to blue divalent ruthenium ions when interacting with sodium amalgam or titanium trichloride.
Safety Profile
Poison by intraperitoneal route. Incompatible with iron pentacarbonyl and zinc. When heated to decomposition it emits toxic fumes of RuO, and Cl-. See also RUTHENIUM COMPOUNDS.
Properties of Ruthenium(III) chloride
| Melting point: | 500 °C |
| Density | 3.11 g/mL at 25 °C (lit.) |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| solubility | insoluble in H2O; slightly soluble in ethanol |
| form | Powder or Crystals |
| color | Black |
| Water Solubility | INSOLUBLE |
| Sensitive | Hygroscopic |
| Merck | 14,8302 |
| Stability: | Stable. Incompatible with zinc. Protect from moisture. |
| InChI | InChI=1S/3ClH.Ru/h3*1H;/q;;;+3/p-3 |
| CAS DataBase Reference | 10049-08-8(CAS DataBase Reference) |
| EPA Substance Registry System | Ruthenium chloride (RuCl3) (10049-08-8) |
Safety information for Ruthenium(III) chloride
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H314:Skin corrosion/irritation H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Ruthenium(III) chloride
| InChIKey | YBCAZPLXEGKKFM-UHFFFAOYSA-K |
| SMILES | [Ru](Cl)(Cl)Cl |
New Products
1-Amino-1-cyclohexanecarboxylic acid 2-Hydroxyacetamide Ethyl 5-cyclopropyl-1H-pyrazole-3-carboxylate 2,4,6-Trichloroaniline ELECTROLYTIC IRON POWDER 1-Aminocyclobutanecarboxylic acid 1-(2-Ethoxyethyl)-2-(piperidin-4-yl)-1H-benzo[d]imidazole hydrochloride Decanonitrile tert-butyl 4-(1H-benzo[d]iMidazol-2-yl)piperidine-1-carboxylate N,N'-diallyl-1,3-diaminopropanedihydrochloride 3-Iodo-6-nitroindazole (4-(4-Fluorophenoxy)phenyl)methanamine Tert-butyl N-(pent-4-yn-2-yl) carbamate 2-Chloro-3-nitropyridine 5-Bromo-2,3-dimethoxypyridine 2-chloro-4-methyl-5-nitro pyridine 2,5-dibromopyridine terephthalonitrile 5-Bromo-4-chloro-2(1h)-pyridinone, 5-Fluoro-2-Oxindole N, N-Carbonyldiimidazole (CDI) 5-Cyanophthalide 10-Methoxy-5H-dibenz[b,f]azepine 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA]Related products of tetrahydrofuran
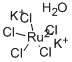
You may like
-
 10049-08-8 Ruthenium trichloride 98%View Details
10049-08-8 Ruthenium trichloride 98%View Details
10049-08-8 -
 10049-08-8 98%View Details
10049-08-8 98%View Details
10049-08-8 -
 Ruthenium Trichloride CAS 10049-08-8View Details
Ruthenium Trichloride CAS 10049-08-8View Details
10049-08-8 -
 Ruthenium(III) chloride, anhydrous, Ru 48% min CAS 10049-08-8View Details
Ruthenium(III) chloride, anhydrous, Ru 48% min CAS 10049-08-8View Details
10049-08-8 -
 Ruthenium trichloride 99%View Details
Ruthenium trichloride 99%View Details -
 Ruthenium trichloride 10049-08-8 99%View Details
Ruthenium trichloride 10049-08-8 99%View Details
10049-08-8 -
 Ruthenium III chloride anhydrous 45-55% Ru content CAS 10049-08-8View Details
Ruthenium III chloride anhydrous 45-55% Ru content CAS 10049-08-8View Details
10049-08-8 -
 Ruthenium(III) chloride CAS 10049-08-8View Details
Ruthenium(III) chloride CAS 10049-08-8View Details
10049-08-8