Benzhydrol
Synonym(s):Benzhydrol;Diphenylmethanol;Diphenyl carbinol;Benzhydryl alcohol;Diphenhydramine Impurity D
- CAS NO.:91-01-0
- Empirical Formula: C13H12O
- Molecular Weight: 184.24
- MDL number: MFCD00004488
- EINECS: 202-033-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:50:25
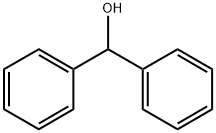
What is Benzhydrol?
Chemical properties
off-white powder or White or colorless crystals. M.P. 69℃. B.p. 281℃. Practically insoluble in water, soluble in alcohol, miscible with oils. The crystals have a very faint musty-rosy odor, somewhat similar to that of Benzophenonc and Trichloromethyl phenyl carbinyl acetate.
The Uses of Benzhydrol
- Benzhydrols are industrially important compounds. On oxidation Benzhydrols yields Benzophenone, which are useful synthones for fullerenes, bioactive oxygen heterocycles, dyes and medicines. Various Cr (VI) and other oxidizing agents are used for oxidizing benzhydrol.
- Benzhydrol is widely used as intermediates in pharmaceuticals (including antihistamines), agrochemicals, perfumes and other organic compounds. It is used as a fixative in the perfume industry. It is involved in polymerization reaction as a terminating group. It is used as precursor to prepare modafinil, benztropine and diphehydramine.
- Intermediate in the preparation of Modafinil (M482500).
What are the applications of Application
Benzhydrol can be used for:
(1) The disproportionation reaction of benzyl alcohol was catalysed using acidic mesoporous Beta (H-MBeta) zeolite under mild conditions to produce equal amounts of diphenylmethane and diphenylketone.
(2) Synthesis of Benzhydrol derivatives as anti-tuberculosis drugs by Friesian rearrangement studied by DSC analysis.
(3) Anchoring of carboxylic acids and alcohols.
Definition
ChEBI: Diphenylmethanol is a secondary alcohol that is diphenylmethane which carries a hydroxy group at position 1. It has a role as a rat metabolite, a bacterial xenobiotic metabolite, a human xenobiotic metabolite and a human urinary metabolite. It is a secondary alcohol and a member of benzyl alcohols. It derives from a hydride of a diphenylmethane.
Preparation
By reduction of Benzophenone, e.g. with zinc in aqueous alkali.
Reactions
Benzhydrol is oxidized to benzophenone, by sodium hypochlorite (commonly known as bleach) in the presence of a phase-transfer catalyst.
Synthesis: In a 20-mL green capped vial, place 1.5 mL of ethyl acetate, 100 mg (0.54 mmol) of benzhydrol and a few drops of methyltricaprylammonium chloride solution (Stark's catalyst or tricaprylmethylammonium chloride). Add a half-inch magnetic stirring bar, and stir until all reagents are dissolved. Cool the solution in an ice bath and add 2 mL of 5% NaOCl (aq) (bleach) dropwise using a 2.5- mL syringe. After the addition of the hypochlorite is complete, allow the reaction to stir for five minutes in the ice-water bath and then stir for a period of one hour at room temperature.

Reduction of benzophenone to benzhydrol.
4MPDC (4-Methyl pyridinium di chromate) is used as oxidizing agent to oxidize benzhydrol. PDC is a mild and selective oxidizing agent and is soluble in water and many organic solvents. Therefore, advantage over inorganic dichromate.
Synthesis Reference(s)
Canadian Journal of Chemistry, 50, p. 3058, 1972 DOI: 10.1139/v72-485
Journal of the American Chemical Society, 55, p. 391, 1933 DOI: 10.1021/ja01328a057
Tetrahedron Letters, 29, p. 139, 1988 DOI: 10.1016/S0040-4039(00)80036-2
General Description
Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards
Purification Methods
Crystallise benzhydrol from hot H2O or pet ether (b 60-70o), pet ether containing a little *benzene, from CCl4, or EtOH (1mL/g). An additional purification step includes passage of a *benzene solution through an activated alumina column. It sublimes in a vacuum. Also recrystallise it three times from MeOH/H2O [Naguib J Am Chem Soc 108 128 1986]. [Beilstein 6 IV 4648.]
Properties of Benzhydrol
| Melting point: | 65-67 °C (lit.) |
| Boiling point: | 297-298 °C (lit.) |
| Density | 1.0120 (rough estimate) |
| vapor pressure | 0.000076 hPa (20 °C) |
| refractive index | 1.5727 (estimate) |
| Flash point: | 160 °C |
| storage temp. | Store below +30°C. |
| solubility | 0.52g/l insoluble |
| form | Crystalline Solid |
| pka | 13.55±0.20(Predicted) |
| color | White to beige |
| Odor | at 100.00 %. weedy green rose |
| Water Solubility | Slightly soluble in water. |
| Merck | 14,1090 |
| BRN | 1424379 |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents, acid chlorides, acid anhydrides, acids. |
| CAS DataBase Reference | 91-01-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzenemethanol, «alpha»-phenyl-(91-01-0) |
| EPA Substance Registry System | Benzenemethanol, .alpha.-phenyl- (91-01-0) |
Safety information for Benzhydrol
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H303:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H320:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for Benzhydrol
Benzhydrol manufacturer
JSK Chemicals
Clickchem Research LLP
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 BENZHYDROL 99%View Details
BENZHYDROL 99%View Details -
 Benzhydrol 98%View Details
Benzhydrol 98%View Details -
 Benzhydrol 98%View Details
Benzhydrol 98%View Details -
 Diphenylcarbinol pure CAS 91-01-0View Details
Diphenylcarbinol pure CAS 91-01-0View Details
91-01-0 -
 Benzhydrol 98% CAS 91-01-0View Details
Benzhydrol 98% CAS 91-01-0View Details
91-01-0 -
 BenzhydrolView Details
BenzhydrolView Details
91-01-0 -
 Benzhydrol (For Synthesis)View Details
Benzhydrol (For Synthesis)View Details
91-01-0 -
 BenzhydrolView Details
BenzhydrolView Details
91-01-0
