Triphenylmethanol
Synonym(s):Triphenyl carbinol;Triphenylcarbinol;Triphenylmethanol;Trityl alcohol
- CAS NO.:76-84-6
- Empirical Formula: C19H16O
- Molecular Weight: 260.33
- MDL number: MFCD00004445
- EINECS: 200-988-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-02 17:37:48
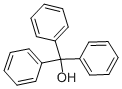
What is Triphenylmethanol?
Chemical properties
white powder or colorless trisolated crystals. Soluble in alcohol, ether and benzene, dark yellow when dissolved in concentrated sulfuric acid, colorless when dissolved in glacial acetic acid, insoluble in water and petroleum ether. Distilled at 360-380℃ without decomposition.
The Uses of Triphenylmethanol
Triphenylmethanol is used as a reagent in the research laboratory. It acts as an intermediate in the production of the commercially useful triarylmethane dyes. It is used in the preparation of triphenylmethane. It is also used as an antiproliferative agent. Further, it is used in the preparation of two-electron reduction product of pyrylogen. In addition to this, it reacts with triphenylphosphine oxide to form a 1:1 molecular complex. It serves as a specific clathrate host for methanol and dimethyl sulphoxide and forms clathrate inclusion complexes.
Triphenylmethanol was used in the synthesis of of the two-electron reduction product of pyrylogen.
It undergoes reduction to triphenylmethane by 9, 10-dihydro-10-methylacridine in the presence of perchloric acid.
The Uses of Triphenylmethanol
Triphenylmethanol (Zidovudine EP Impurity D) is a triaryl methane derivative as antiproliferative agent.
Preparation
Triphenylmethanol synthesis: Triphenylmethanol was prepared by the action of benzene with carbon tetrachloride in the presence of Aluminum chloride, followed by acidification and hydrolysis.
Triphenylmethanol can also be prepared by the reaction of phenylmagnesium bromide with methyl benzoate (instead of benzophenone).
Synthesis of Triphenylmethanol
Reactions
The first one is the formation of the triphenylmethyl bromide from the reaction of triphenylmethanol with hydrobromic acid.
The second reaction is the formation of an ether from the reaction of triphenylmethanol with methanol in acidic conditions.
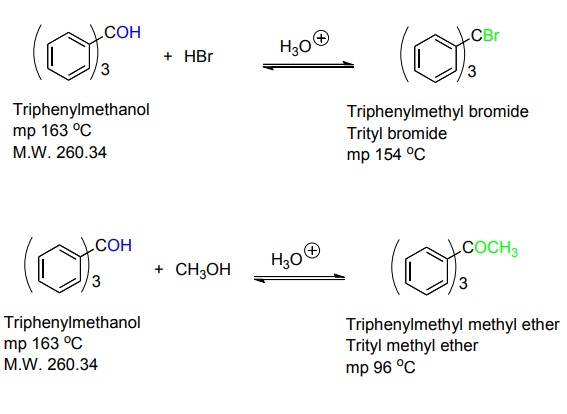
Synthesis Reference(s)
Organic Syntheses, Coll. Vol. 3, p. 839, 1955
The Journal of Organic Chemistry, 57, p. 4555, 1992 DOI: 10.1021/jo00042a044
General Description
Triphenylmethanol forms 1:1 molecular complex with triphenylphosphine oxide. It is a specific clathrate host for methanol and dimethyl sulphoxide and forms clathrate inclusion complexes. It undergoes reduction to triphenylmethane by 9, l0-dihydro-10-methylacridine in the presence of perchloric acid.
Purification Methods
Crystallise the carbinol from EtOH, MeOH, CCl4 (4mL/g), *benzene, hexane or pet ether (b 60-70o). Dry it at 90o. [Ohwada et al. J Am Chem Soc 108 3029 1986, Beilstein 6 IV 5014.]
Properties of Triphenylmethanol
| Melting point: | 160-163 °C (lit.) |
| Boiling point: | 360 °C (lit.) |
| Density | d40 1.199 |
| refractive index | 1.6220 (estimate) |
| Flash point: | 360-380°C |
| storage temp. | 2-8°C |
| solubility | dioxane: 0.1 g/mL, clear |
| form | Fine Crystalline Powder |
| pka | 12.73±0.29(Predicted) |
| color | White to light yellow |
| Water Solubility | INSOLUBLE |
| Merck | 14,9739 |
| BRN | 1460837 |
| Stability: | Stable. Combustible. Incompatible with oxidizing agents, acids, acid chlorides, acid anhydrides. |
| CAS DataBase Reference | 76-84-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzenemethanol, «alpha»,«alpha»-diphenyl-(76-84-6) |
| EPA Substance Registry System | Benzenemethanol, .alpha.,.alpha.-diphenyl- (76-84-6) |
Safety information for Triphenylmethanol
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for Triphenylmethanol
Triphenylmethanol manufacturer
Clickchem Research LLP
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 76-84-6 97.94View Details
76-84-6 97.94View Details
76-84-6 -
 Triphenylcarbinol CAS 76-84-6View Details
Triphenylcarbinol CAS 76-84-6View Details
76-84-6 -
 Triphenylmethanol 98% CAS 76-84-6View Details
Triphenylmethanol 98% CAS 76-84-6View Details
76-84-6 -
 Triphenylmethanol CAS 76-84-6View Details
Triphenylmethanol CAS 76-84-6View Details
76-84-6 -
 Triphenylmethanol CAS 76-84-6View Details
Triphenylmethanol CAS 76-84-6View Details
76-84-6 -
 Triphenylmethanol CAS 76-84-6View Details
Triphenylmethanol CAS 76-84-6View Details
76-84-6 -
 Triphenylmethanol CAS 76-84-6View Details
Triphenylmethanol CAS 76-84-6View Details
76-84-6 -
 Triphenyl methanol Cas no : 76-84-6View Details
Triphenyl methanol Cas no : 76-84-6View Details
76-84-6
