Bazedoxifene
- CAS NO.:198481-32-2
- Empirical Formula: C30H34N2O3
- Molecular Weight: 470.6
- MDL number: MFCD05148441
- EINECS: 805-732-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-10-31 12:04:19
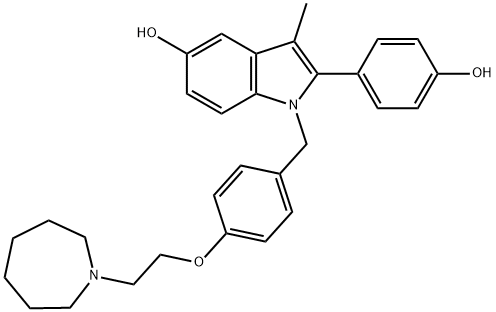
What is Bazedoxifene?
Absorption
Bazedoxifene is rapidly absorbed with a tmax of approximately 2 hours and exhibits a linear increase in plasma concentrations for single doses from 0.5 mg up to 120 mg and multiple daily doses from 1 mg to 80 mg. The absolute bioavailability of bazedoxifene is approximately 6%.
The Uses of Bazedoxifene
Bazedoxifene is an nonsteroidal selective estrogen receptor modulator (SERM). Bazedoxifene is used as an antiosteoporotic.
Background
Bazedoxifene is a third generation selective estrogen receptor modulator (SERM), developed by Pfizer following the completion of their takeover of Wyeth Pharmaceuticals. In late 2013, Pfizer received approval for bazedoxifene as part of the combination drug DUAVEE in the prevention (not treatment) of postmenopausal osteoporosis. It is approved in the European Union (marketed in Italy and Spain) and Japan as monotherapy. In 2013, the combination product containing conjugated estrogens and bazedoxifene was approved by the FDA for the treatment of moderate to severe vasomotor symptoms associated with menopause, as well as the prevention of postmenopausal osteoporosis in women.
Indications
Indicated for following conditions alone or in combination with conjugated estrogens in women with a uterus:
Definition
ChEBI: Bazedoxifene is a phenylindole.
General Description
Bazedoxifene, 1-[[4-[2-(azepan-1-yl)ethoxy]phenyl]methyl]-2-(4-hydroxyphenyl)-3-methylindol-5-ol (Viviant), was declared to be “approvable” bythe FDA in 2007 for the prevention of postmenopausalosteoporosis. As of mid-2009, final approval in the UnitedStates is still pending. Bazedoxifene was approved inEurope in 2009 for the treatment of post–menopausalosteoporosis.
Clinical Use
Bazedoxifene is an indole-based SERM that is under investigation for the treatment and prevention of postmenopausal osteoporosis. It also is being evaluated in combination with Premarin (conjugated estrogens). Bazedoxifene displaces 17β-estradiol from estrogen receptors and has excellent binding affinity for the receptor itself. Unlike raloxifene, this agent does not cause hot flashes at the doses required to have a beneficial effect on bone. In addition, it does not cause uterine or mammary gland stimulation.
Enzyme inhibitor
This potent and selective estrogen receptor modulator, or SERM (FW = 530.65 g/mol; CAS 198481-33-3; Solubility: 00 mM in DMSO), also named 1-[[4-[2- (hexahydro-1H-azepin-1-yl) ethoxy]phenyl]methyl]-2- (4- hydroxyphenyl) -3-methyl-1H-indol-5-ol, selectivity targets the extrogen receptor ERα (IC50 = 26 nM), with weaker action against ERβ (IC50 = 99 nM), inhibiting 17β-estradiol-induced proliferation of MCF-7 cells. Bazedoxifene represents a promising new treatment for osteoporosis, one with a potential for less uterine and vasomotor effects than selective estrogen receptor modulators now used clinically.
Metabolism
Glucuronidation is the major metabolic pathway. After peroral application, bazedoxifene is metabolized by UDP-glucuronosyltransferases (UGTs) to bazedoxifene-4'-glucuronide (M4) and bazedoxifene-5-glucuronide (M5).Little or no cytochrome P450-mediated metabolism is evident. The concentrations of this glucuronide are approximately 10-fold higher than those of unchanged active substance in plasma.
Properties of Bazedoxifene
| Melting point: | 98-102° |
| Boiling point: | 694.4±55.0 °C(Predicted) |
| Density | 1.19 |
| storage temp. | Sealed in dry,Store in freezer, under -20°C |
| solubility | Chloroform (Slightly), DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 10.12±0.15(Predicted) |
| color | White to Very Dark Beige |
Safety information for Bazedoxifene
| Signal word | Danger |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H360:Reproductive toxicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P281:Use personal protective equipment as required. P308+P313:IF exposed or concerned: Get medical advice/attention. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for Bazedoxifene
Bazedoxifene manufacturer
Aarti Industries Limited (AIL)
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![1-[[4-[2-(Hexahydro-1-oxido-1H-azepin-1-yl)ethoxy]phenyl]Methyl]-2-(4-hydroxyphenyl)-3-Methyl-1H-indol-5-ol](https://img.chemicalbook.in/CAS/GIF/1174289-22-5.gif)

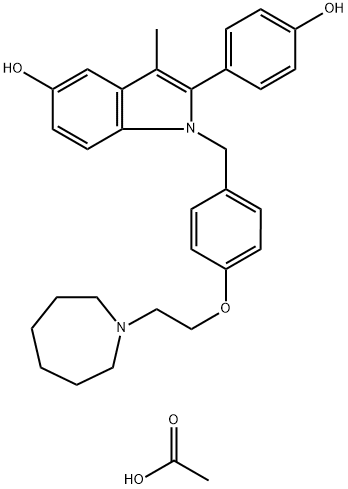
You may like
-
 198481-32-2 Bazedoxifene 98%View Details
198481-32-2 Bazedoxifene 98%View Details
198481-32-2 -
 198481-32-2 98%View Details
198481-32-2 98%View Details
198481-32-2 -
 Bazedoxifene 98%View Details
Bazedoxifene 98%View Details
198481-32-2 -
 198481-32-2 98%View Details
198481-32-2 98%View Details
198481-32-2 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1