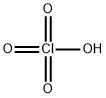
BARIUM PERCHLORATE
Synonym(s):Barium diperchlorate;Barium perchlorate;Perchloric acid barium salt
- CAS NO.:13465-95-7
- Empirical Formula: BaClH3O4
- Molecular Weight: 239.8
- MDL number: MFCD00003440
- EINECS: 236-710-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
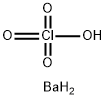
What is BARIUM PERCHLORATE?
Description
Barium perchlorate is a white crystalline solid.Molecular weight=336.2; Freezing/Melting point=400℃(trihydrate); 505℃ (anhydrous). Hazard Identification(based on NFPA-704 M Rating System): Health 2,Flammability 0, Reactivity 0. Highly soluble in water;solubility=200 g/100 mL at 33℃
Chemical properties
White Crystalline Powder
The Uses of BARIUM PERCHLORATE
Barium perchlorate, anhydrous is used to make explosives. It is also used as desiccant, ribonuclease determination.
The Uses of BARIUM PERCHLORATE
Ribonuclease determination and effective desiccant.
The Uses of BARIUM PERCHLORATE
In the determination of ribonuclease; as absorbent for water in C and H analysis.
General Description
A white crystalline solid. Noncombustible, but accelerates burning of combustible materials. May explode if large quantities are involved in a fire or the combustible material is finely divided. Prolonged exposure to fire or heat may result in an explosion. Used to make explosives.
Air & Water Reactions
Water soluble.
Reactivity Profile
Reflux heating of BARIUM PERCHLORATE with alcohols yields perchlorate esters, which are highly explosive [Kirk-Othmer 2nd ed. 5:75 1963]. A strong oxidizing agent. May react violently with organic materials.
Health Hazard
Inhalation or contact with eyes or skin causes irritation. Ingestion causes excessive salivation, vomiting, colic, diarrhea, convulsive tremors, slow, hard pulse, and elevated blood pressure; hemorrhages may occur in the stomach, intestines, and kidneys; muscular paralysis may follow.
Fire Hazard
Behavior in Fire: Increases the intensity of fire. Containers may explode.
Safety Profile
A poison. An unstable material. An oxidizer. When refluxed with an alcohol highly explosive alkyl perchlorates are formed. When heated to decomposition it emits toxic fumes of Cl-. A desiccant. See also PERCHLORATES and BARIUM COMPOUNDS.
Potential Exposure
It is used to make explosives and inexperimental rocket fuels.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seekmedical attention immediately. If this chemical contactsthe skin, remove contaminated clothing and wash immediately with soap and water. Seek medical attention immediately. If this chemical has been inhaled, remove fromexposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing hasstopped and CPR if heart action has stopped. Transferpromptly to a medical facility. When this chemical hasbeen swallowed, get medical attention. Give large quantities of water and induce vomiting. Do not make an unconscious person vomit.
Storage
Color Comables and combustibles. Store in tightly closed containersin a cool, well-ventilated area away from heat sources,sources of shock, or the incompatible materials cited above.Sources of ignition, such as smoking and open flames, areprohibited where Barium perchlorate is handled, used, orstored. See OSHA Standard 1910.104 and NFPA 43A Codefor the Storage of Liquid and Solid Oxidizers for detailedhandling and storage regulationsde—Yellow: Reactive Hazard; Store in alocation separate from other materials, especially flam
Shipping
Barium perchlorate requires a shipping labelof “OXIDIZER, POISONOUS/TOXIC MATERIALS.”This material falls in Hazard Class 5.1 and PackingGroup II.[19, 20]
Purification Methods
Recrystallise the perchlorate twice from water. [Schmeisser in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 320 1963.]
Incompatibilities
An oxidizing agent. Contact with organicand combustible materials (such as paper, wood, and oil),finely divided metals (specifically magnesium and aluminum), sulfur, calcium hydride, and strontium hydride sinceviolent reactions occur
Properties of BARIUM PERCHLORATE
| Melting point: | 505 °C(lit.) |
| Density | 3.2 g/mL at 25 °C(lit.) |
| Flash point: | 21 °C |
| storage temp. | Store at +5°C to +30°C. |
| solubility | 1985g/l |
| form | Powder |
| Specific Gravity | 3.2 |
| color | White |
| PH | 5.6 (100g/l, H2O, 20℃) |
| Odor | Odorless |
| Water Solubility | Soluble in water. |
| Sensitive | Hygroscopic |
| Merck | 14,987 |
| Exposure limits | ACGIH: TWA 0.5 mg/m3 NIOSH: IDLH 50 mg/m3; TWA 0.5 mg/m3 |
| CAS DataBase Reference | 13465-95-7(CAS DataBase Reference) |
| EPA Substance Registry System | Perchloric acid, barium salt (2:1) (13465-95-7) |
Safety information for BARIUM PERCHLORATE
| Signal word | Danger |
| Pictogram(s) |
 Flame Over Circle Oxidizers GHS03  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H271:Oxidising liquids;Oxidising solids |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. |
Computed Descriptors for BARIUM PERCHLORATE
BARIUM PERCHLORATE manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 barium perchlorate anhydrous 99%View Details
barium perchlorate anhydrous 99%View Details -
 Barium perchlorate CAS 13465-95-7View Details
Barium perchlorate CAS 13465-95-7View Details
13465-95-7 -
 Barium perchlorate CAS 13465-95-7View Details
Barium perchlorate CAS 13465-95-7View Details
13465-95-7 -
 Barium perchlorate, GR 98% CAS 13465-95-7View Details
Barium perchlorate, GR 98% CAS 13465-95-7View Details
13465-95-7 -
 Barium perchlorate solution CASView Details
Barium perchlorate solution CASView Details -
 Barium Perchlorate CAS No. 13465-95-7.View Details
Barium Perchlorate CAS No. 13465-95-7.View Details
13465-95-7 -
 Barium Perchlorate Ar (anhydrous)View Details
Barium Perchlorate Ar (anhydrous)View Details
13465-95-7 -
 Barium PerchlorateView Details
Barium PerchlorateView Details
13465-95-7
