Barium acetate
Synonym(s):Barium acetate
- CAS NO.:543-80-6
- Empirical Formula: C4H6BaO4
- Molecular Weight: 255.42
- MDL number: MFCD00012447
- EINECS: 208-849-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
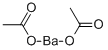
What is Barium acetate?
Description
Barium acetate (Ba(C2H3O2)2) is the salt of barium (II) and acetic acid.
Chemical properties
White Crystalline Powder
Chemical properties
Barium acetate is a white powder, which is highly soluble: at 0 °C, 55.8 g of barium acetate can be dissolved in 100 g of water. It decomposes upon heating into barium carbonate .
Physical properties
White powdery solid; density 2.47g/cm3; decomposes on heating; highly soluble in water (55.8g /100g at 0°C), sparingly soluble in methanol (~1.43 g per liter).
The Uses of Barium acetate
Barium acetate is used as a mordant for printing textile fabrics, for drying paints and varnishes and in lubricating oil. In chemistry, it is used in the preparation of other acetates; and as a catalyst in organic synthesis.
The Uses of Barium acetate
Used as catalyst for organic reactions
The Uses of Barium acetate
Barium acetate [Ba(C2H3O2)2?H2O], a white crystal, is used as a dryer for paints and varnishes. It is produced by adding acetic acid to barium sulfate and recovering the crystals by evaporation. It is also used as a textile mordant and catalyst.
What are the applications of Application
Barium acetate is a component in various biological assays
Preparation
Barium acetate is generally produced by the reaction of acetic acid with barium carbonate :
BaCO3 + 2 CH3COOH → (CH3COO)2Ba + CO2 + H2O
The reaction is performed in solution and the barium acetate crystallizes out. Alternatively, barium sulfide can be used :
BaS + 2 CH3COOH → (CH3COO)2Ba +H2S
Again, the solvent is evaporated off and the barium acetate crystallized.
Definition
ChEBI: Barium acetate is an acetate salt in which the cationic component is barium(2+). It has a role as a mordant and a catalyst. It is an organic barium salt and an acetate salt.
Reactions
When heated in air, barium acetate decomposes to the carbonate. It reacts with acids: reaction with sulfuric acid, hydrochloric acid and nitric acid give the sulfate, chloride and nitrate respectively.
General Description
Barium acetate is the barium salt of acetic acid.
Hazard
The salt or its aqueous solution is highly toxic. LD10 (oral) rabbit: 236 mg/kg; LD10 (subcutaneous) rabbit: 96 mg/kg. See Barium.
Flammability and Explosibility
Not classified
Safety Profile
Poison via ingestion, intravenous, and subcutaneous routes. When heated to decomposition it emits acrid smoke and fumes. See also BARIUM COMPOUNDS.
Purification Methods
Crystallise the salt twice from anhydrous acetic acid and dry it under vacuum for 24hours at 100o. [Beilstein 2 I 49, 2 II 117, 2 III 192, 2 IV 114.]
Properties of Barium acetate
| Melting point: | 450°C |
| Density | 2.46 |
| vapor pressure | 15.7hPa at 25℃ |
| storage temp. | Store at +5°C to +30°C. |
| solubility | 720g/l |
| form | Solid |
| color | White |
| Specific Gravity | 2.468 |
| PH Range | 7 - 8.5 at 50 g/l at 25 °C |
| PH | 7-8.5 (20℃, 5%) |
| Odor | Slight acetic acid odor |
| Water Solubility | soluble |
| Merck | 14,965 |
| BRN | 3693411 |
| Exposure limits | ACGIH: TWA 0.5 mg/m3 NIOSH: IDLH 50 mg/m3; TWA 0.5 mg/m3 |
| Stability: | Stable. Incompatible with strong oxidizing agents, acids. |
| CAS DataBase Reference | 543-80-6(CAS DataBase Reference) |
| EPA Substance Registry System | Barium acetate (543-80-6) |
Safety information for Barium acetate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P271:Use only outdoors or in a well-ventilated area. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. |
Computed Descriptors for Barium acetate
| InChIKey | ITHZDDVSAWDQPZ-UHFFFAOYSA-L |
Barium acetate manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
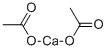

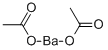
You may like
-
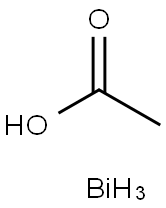
 Barium acetate 98%View Details
Barium acetate 98%View Details -
 Barium acetate CAS 543-80-6View Details
Barium acetate CAS 543-80-6View Details
543-80-6 -
 Barium acetate CAS 543-80-6View Details
Barium acetate CAS 543-80-6View Details
543-80-6 -
 Barium acetate CAS 543-80-6View Details
Barium acetate CAS 543-80-6View Details
543-80-6 -
 Barium acetate CAS 543-80-6View Details
Barium acetate CAS 543-80-6View Details
543-80-6 -
 Barium acetate CAS 543-80-6View Details
Barium acetate CAS 543-80-6View Details
543-80-6 -
 Barium acetate, 98% 99%View Details
Barium acetate, 98% 99%View Details -
 Barium AcetateView Details
Barium AcetateView Details
543-80-6
