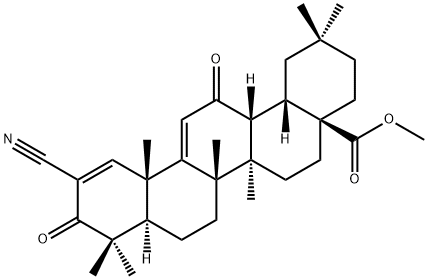
Bardoxolone methyl
- CAS NO.:218600-53-4
- Empirical Formula: C32H43NO4
- Molecular Weight: 505.69
- MDL number: MFCD11983137
- EINECS: 606-850-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
What is Bardoxolone methyl?
Description
Bardoxolone methyl is an antioxidant inflammatory modulator that reversibly interacts with critical free thiol groups of cysteine residues on KEAP-1 and other target proteins, inducing the trans-location of Nrf-2 to the nucleus and the activation of several genes, including glutathione S-transferase, haeme oxygenase, and other components of the cytoprotective response (Thomas et al., 2012). At high doses, bardoxolone methyl may also interact with other proteins, including PPAR-gamma, tubulin, and the kinase activa-tor IKK.
Chemical properties
White to Off-White Solid
The Uses of Bardoxolone methyl
Bardoxolone methyl is an antiinflam-matory drug that activates the Nrf2-Keap1 pathway,resulting in inhibition of the proinflammatory cytokine Nf-κB.
Bardoxolone methyl (BARD, CDDO-ME, RTA-402) is an orally bioavailable semi-synthetic triterpenoid compound based of the scaffold of oleanolic acid. It was developed by Reata Pharmaceuticals as an activator of the Nrf2 antioxidant pathway. It has been tested in clinical trials for kidney disease, pulmonary hypertension, and cancer.
The Uses of Bardoxolone methyl
CDDO Methyl Ester is a synthetic triterpenoid that inhibits IκBα kinase and enhances apoptosis induced by TNF and chemotherapeutic agents through down-regulation of expression of nuclear factor κB-regulated gene products in human leukemic cells. CDDO Methyl Ester is a novel therapeutic agent in the treatment of acute myeloid leukemia and in the treatment of pancreatic cancer as well as other forms of cancer.
Definition
ChEBI: Bardoxolone methyl is a member of cyclohexenones.
Clinical Use
Bardoxolone methyl is a synthetic compound derived from oleanolic acid,whichactivates the Keap1-Nrf2 pathway and regulates inflammation in the kidney. In the Bardoxolone Methyl Treatment: Renal Function in CKD/Type 2 Diabetes (BEAM)study,patients with CKD and diabetes wererandomly assigned to receive either bardoxolone methyl or placebo for 52 weeks.Bardoxolone methyl significantly increased the mean eGFR compared with placebo at 24 weeks. The improvement lasted for another 28 weeks. Adverse events, particularly muscle spasms,were more frequent in the bardoxolone methyl group.The Bardoxolone Methyl Evaluation in Patients with Chronic Kidney Disease and Type 2 Diabetes Mellitus: the Occurrence of Renal Events study was designed to confirm the findings of the BEAM study. Unfortunately the study was prematurely stopped owing to unacceptable high rates of cardiovascular events in patients treated with bardoxolone methyl at a medianduration of 7 months,and no benefit wasobserved about the risk of ESRD. The beneficial effects of an Nrf2 agonist called dh404, which is a derivative of bardoxolone methyl, via reduction in inflammation and oxidative stress but only at low doses have recently been shown in mice. This finding rekindles the interests on renoprotection via activation of the Nrf2 pathway in DKD.
Enzyme inhibitor
This oleanane triterpenoid (FW = 505.70 g/mol; CAS 218600-53-4; Solubility: 1 mg/mL DMSO; <1 mg/mL H2O), also known as RTA 402, TP- 155, NSC 713200, CDDO Methyl Ester, and 2-cyano-3,12-dioxooleana- 1,9(11)-dien-28-oic acid methyl ester, targets Inhibitor of nuclear factor Kappa-B Kinase subunit b, or IKKb, a key component of the cytokine activated intracellular signaling pathway that triggers immune responses. Significantly, CDDO methyl ester inhibits proliferation of myeloid leukemia cells, inducing both differentiation and apoptosis. CDDO-Me induces loss of mitochondrial membrane potential, induction of caspase-3 cleavage, increase in annexin V binding, and DNA fragmentation – all suggesting induction of apoptosis. CDDO-Me induces pro-apoptotic Bax protein that preceded caspase activation. CDDO-Me also inhibits ERK1/2 activation, as indicated by inhibition of mitochondrial ERK1/2 phosphorylation and blocking of Bcl-2 phosphorylation, rendering the latter less anti-apoptotic. CDDO-Me inhibits NF-kB through inhibition of IkBa kinase, resulting in the suppression of expression of NF-kB-regulated gene products (Readouts: IAP2, cFLIP, TRAF1, survivin, and Bcl-2) as well as inhibition of proliferation (Readouts: cyclin d1 and c-myc), and angiogenesis (Readouts: VEGF, Cox-2, and MMP-9). CDDO-Me thus enhances apoptosis induced by TNF and chemotherapeutic agents. Bardoxolone methyl exhibits potent pro-apoptotic and anti-inflammatory activity, potently inhibiting interferon γ-induced nitric oxide synthesis in mouse macrophages, IC50 = 0.1 nM. Bardoxolone methyl pretreatment uniquely confers protection against lipopolysaccharide (LPS) challenge by modulating the in vivo immune response to LPS, indicating that it represents a novel oral agent for use in LPS-mediated inflammatory diseases. Significantly, a number of well-recognized naturally occurring or synthetic anti-inflammatory compounds possessing a Michael-type acceptor (e.g., thymoquinone (TQ), the paracetamol metabolite NAPQI, the 5-LO inhibitor AA-861, and bardoxolone methyl) are all direct covalent 5-LO enzyme inhibitors that target the catalytically relevant Cysteines 416 and 418. Their actiom as irreversible Michael acceptor moietied that interact with required cysteines of proteins/enzymes may expain why they are effective and sustained enzyme activity modulators.
storage
Store at -20°C
Mode of action
Bardoxolone Methyl is the methyl ester form of bardoxolone, a synthetic triterpenoid compound with potential antineoplastic and anti-inflammatory activities. Bardoxolone blocks the synthesis of inducible nitric oxide synthase (iNOS) and inducible cyclooxygenase (COX-2), two enzymes involved in inflammation and carcinogenesis. This agent also inhibits the interleukin-1 (IL-1)-induced expression of the pro-inflammatory proteins matrix metalloproteinase-1 (MMP-1) and matrix metalloproteinase-13 (MMP-13) and the expression of Bcl-3; Bcl-3 is an IL-1-responsive gene that preferentially contributes to MMP-1 gene expression.
Properties of Bardoxolone methyl
| Melting point: | 215-223°C |
| Boiling point: | 600.8±55.0 °C(Predicted) |
| Density | 1.15 |
| storage temp. | Refrigerator |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | Solid |
| color | White to Light Brown |
| CAS DataBase Reference | 218600-53-4 |
Safety information for Bardoxolone methyl
Computed Descriptors for Bardoxolone methyl
| InChIKey | WPTTVJLTNAWYAO-KPOXMGGZSA-N |
| SMILES | C1(=O)[C@@]([C@]2([C@](C)(C=C1C#N)C1[C@@]([C@]3([C@@](C(=O)C=1)([H])[C@]1([C@@](CC[C@](C1)(C)C)(C(OC)=O)CC3)[H])C)(C)CC2)[H])(C)C |
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 CDDO Methyl Ester CAS 218600-53-4View Details
CDDO Methyl Ester CAS 218600-53-4View Details
218600-53-4 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1