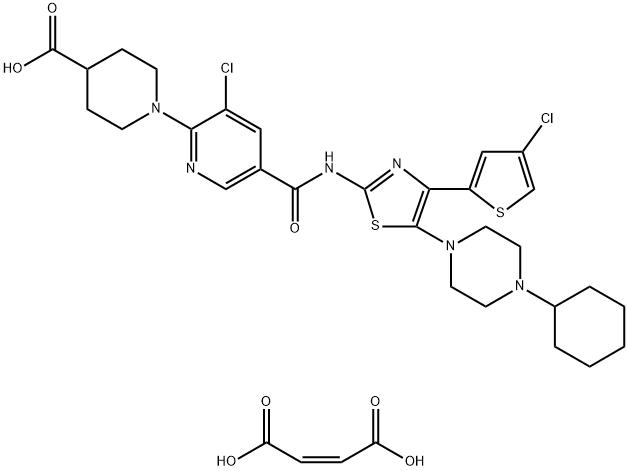
Avatrombopag maleate
- CAS NO.:677007-74-8
- Empirical Formula: C33H38Cl2N6O7S2
- Molecular Weight: 765.72
- MDL number: MFCD12032126
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-28 16:48:35
What is Avatrombopag maleate?
Description
Avatrombopag maleate is a thrombopoietin receptor (TPO) agonist developed by AkaRx Inc and approved by the US FDA in May 2018 as Doptelet (20mg). For the treatment of low platelet count (thrombocytopenia CIT) in adult patients with chronic liver disease (CLD) who are scheduled to undergo medical or dental surgery.
Chemical properties
Avatrombopag maleate drug substance is a white to off-white, non-hygroscopic, powder that is practically insoluble in water and in 0.1 M HCl. The aqueous solubility of avatrombopag maleate at various pH levels indicates that the drug substance is practically insoluble at pH 1 to 11. Avatrombopag maleate drug substance is an achiral molecule.
The Uses of Avatrombopag maleate
Avatrombopag Maleate is the maleate salt form of avatrombopag, an orally available platelet thrombopoietin receptor (TPOR; MPL) agonist, with potential megakaryopoiesis stimulating activity.
Definition
Avatrombopag maleate is an active ingredient in Doptelet. Doptelet is provided as an immediate-release tablet. Each DOPTELET tablet contains 20 mg avatrombopag (equivalent to 23.6 mg avatrombopag maleate). Other components of Doptelet include lactose monohydrate, colloidal silicon dioxide, crospovidone, and so on.
Mechanism of action
Upon administration, Avatrombopag maleate binds to and stimulates TPOR, which may lead to the proliferation and differentiation of megakaryocytes from bone marrow progenitor cells. This increases the production of platelets and may prevent chemotherapy-induced thrombocytopenia (CIT). TPOR is a cytokine receptor and member of the hematopoietin receptor superfamily.
Synthesis
The synthesis of Avatrombopag maleate is as follows:
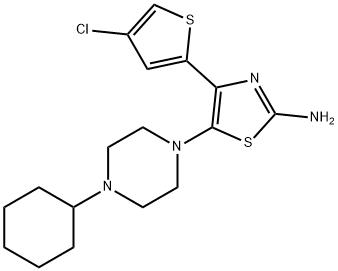
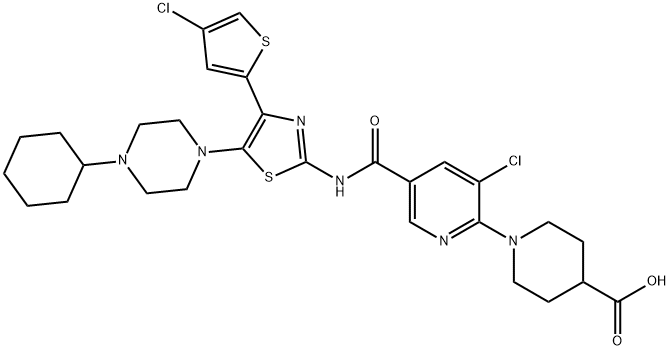
A mixture containing 80.1 g of 1-(3-chloro-5-{[4-(4-chlorothiophen-2-yl)-5-(4-cyclohexyl piperazin-1-yl) thiazol-2-yl] carbamoyl} pyridin-2-yl) piperidine-4-carboxylic acid, 580 mL of DMSO, 580 mL of acetone, 17.2 g of maleic acid, and 290 mL of water was stirred at 69 °C. The insoluble matter was filtered out, washed with a mixture of 32 mL of DMSO, 32 mL of acetone, and 16 mL of water, and then the filtrate was cooled and the precipitate was collected by filtering. Washing was successively performed using 150 mL of water, 80 mL of acetone, 650 mL of water, and 80 mL of acetone, followed by drying, to obtain 70.66 g of Avatrombopag maleate.
Safety information for Avatrombopag maleate
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![methyl 3'-amino-2'-hydroxy-[1,1'-biphenyl]-3-carboxylate](https://img.chemicalbook.in/CAS/20200611/GIF/2230800-88-9.gif)
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1