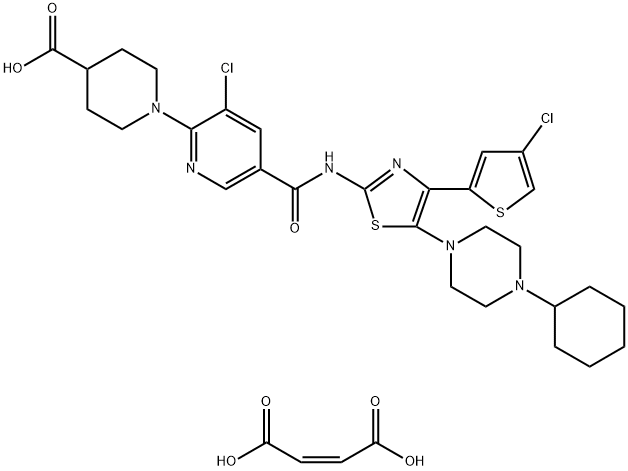
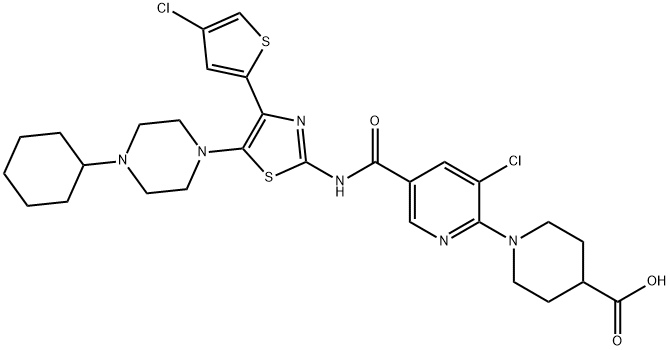
Lusutrombopag
- CAS NO.:1110768-00-7
- Empirical Formula: C31H36Cl2N2O5S
- Molecular Weight: 619.6
- MDL number: MFCD28502075
- Update Date: 2023-07-14 17:33:20
What is Lusutrombopag?
Description
Lusutrombopag is an orally bioavailable thrombopoietin (TPO) receptor agonist developed by Shionogi for improvement of thrombocytopenia associated with chronic liver disease in patients undergoing an elective invasive procedure (e.g., liver biopsy, liver transplantation). Thrombocytopenia, which is common among patients with chronic liver disease, increases the risk of bleeding when undergoing invasive procedures, which in turn complicates therapy and increases the risk of mortality. Lusutrombopag, which was approved in Japan in September 2015, promotes platelet production by stimulating the proliferation and differentiation of human bone marrow progenitor cells into megakaryocytes via the thrombopoietic pathway. The consequent increase in platelet levels avoids postponement of invasive procedures or transfusion of platelets and administration of platelet products, the current standard of care for thrombocytopenia in these patients.
The Uses of Lusutrombopag
Lusutrombopag is a newly discovered thrombopoietin receptor agonist used in the treatment of patients with chronic ITP.
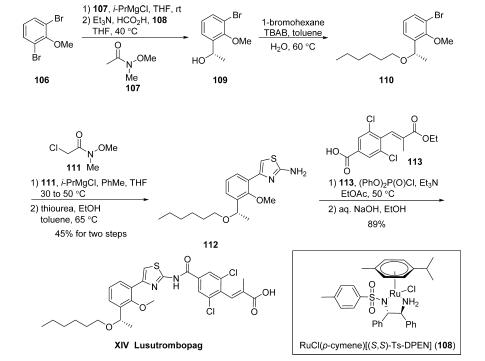
Synthesis
To date, only two synthetic routes to lusutrombopag have
been reported: one in the Japanese patent literature which has
been exemplified on kilogram scale and the other a closely
related discovery route which has been reported in the United
States patent literature. Commercial 2,6-dibromoanisole
(106) was treated with isopropylmagnesium chloride to form
the corresponding Grignard reagent prior to reaction with
Weinreb amide 107, furnishing a ketone which underwent
immediate reduction with formic acid in the presence of chiral
catalyst RuCl(p-cymene)[(S,S)-Ts-DPEN] (108) and generate
the desired (S)-stereogenic alcohol 109. 
Unfortunately, neither
the yield nor the stereoselectivity of this reduction was reported
in any of the disclosures. Benzyl alcohol 109 was subjected to
Williamson etherification conditions with n-hexyl bromide to
furnish ether 110. The aryl bromide within 110 was then
converted to the corresponding Grignard reagent, which was
reacted with N-methyloxy-N-methyl-2-chloroacetamide (111),
followed by subsequent treatment with thiourea in toluene/
ethanol at elevated temperatures to give aminothiazole
intermediate 112 in 45% yield across the two-step sequence.
Next, activation of acid 113 prior to exposure to 112 facilitated
amide bond formation. Saponification of the pendant ester with
sodium hydroxide furnished luxutrombopag (XIV) in 89%
yield. Although acid 113 is not commercial, it could be
prepared from 3,5-dichlorobenzoic acid (33) via formylation
with 4-formylmorpholine, followed by a Horner-Wadsworth-Emmons reaction with triethylphosphonopropionate.
Properties of Lusutrombopag
| Melting point: | 184 - 186°C |
| Density | 1.246±0.06 g/cm3(Predicted) |
| storage temp. | Refrigerator, under inert atmosphere |
| solubility | Chloroform (Slightly), DMSO (Slightly) |
| form | Solid |
| pka | 6.30±0.50(Predicted) |
| color | White Off-White |
Safety information for Lusutrombopag
Computed Descriptors for Lusutrombopag
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde Electrolytic Iron Powder 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 4-HYDROXY BENZYL ALCOHOL 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) S-2-CHLORO PROPIONIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 3-(2,4-Dimethoxybenzyl)dihydropyrimidine-2,4(1H,3H)-dione 6-Bromo-3-iodo-1-methyl-1H-indazole 4-Ethylbenzylamine N-(5-Amino-2-methylphenyl)acetamide 2-(BOC-Amino)4-picoline 1-(4-Methylphenylsulfonyl)-1H-1,2,3-benzotriazoleRelated products of tetrahydrofuran
You may like
-
 100-71-0 99%View Details
100-71-0 99%View Details
100-71-0 -
 2 2-BIS(2-HYDROXYETHOXY)-1 1-BINAPHTHYL 99%View Details
2 2-BIS(2-HYDROXYETHOXY)-1 1-BINAPHTHYL 99%View Details
55441-95-7 -
 Chloro Uracil 1820-81-1 99%View Details
Chloro Uracil 1820-81-1 99%View Details
1820-81-1 -
 181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1 -
 2-ethyl-6-methyl-3-hydroxypyridine succinate 127464-43-1 99%View Details
2-ethyl-6-methyl-3-hydroxypyridine succinate 127464-43-1 99%View Details
127464-43-1 -
 13162-05-5 N-Vinylformamide 99%View Details
13162-05-5 N-Vinylformamide 99%View Details
13162-05-5 -
 1446013-08-6 98%View Details
1446013-08-6 98%View Details
1446013-08-6 -
 Ste-Glu-AEEA-AEEA-OSUView Details
Ste-Glu-AEEA-AEEA-OSUView Details
1169630-40-3
![methyl 3'-amino-2'-hydroxy-[1,1'-biphenyl]-3-carboxylate](https://img.chemicalbook.in/CAS/20200611/GIF/2230800-88-9.gif)