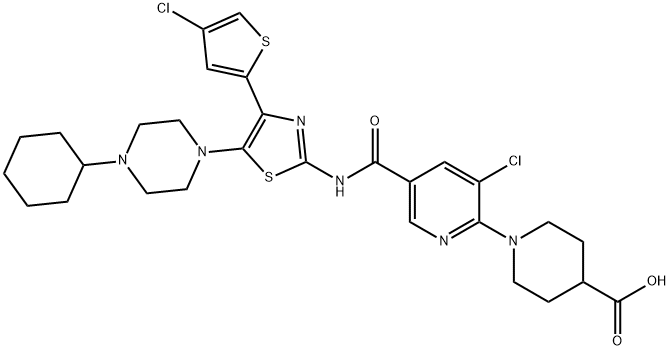
Avatrombopag
- CAS NO.:570406-98-3
- Empirical Formula: C29H34Cl2N6O3S2
- Molecular Weight: 649.65
- MDL number: MFCD18633249
- EINECS: 1312995-182-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 20:33:22
What is Avatrombopag?
Absorption
Following single dosing under fasted and fed conditions, mean peak concentrations occurred at 5-8 hours and declined with a half-life of 16-18 hours in Japanese and white subjects. Administration with food did not have an effect on the rate or extent of avatrombopag absorption, however, significantly reduced pharmacokinetic variability relative to the fasting state .
Avatrombopag showed dose-proportional pharmacokinetics after single doses from 10 mg (0.25-times the lowest approved dosage) to 80 mg (1.3-times the highest recommended dosage). Healthy subjects administered 40 mg of avatrombopag showed a geometric mean (%CV) maximal concentration (Cmax) of 166 (84%) ng/mL and area under the time-concentration curve, extrapolated to infinity (AUC0-inf) of 4198 (83%) ng.hr/mL. The pharmacokinetics of avatrombopag are similar in both healthy subjects and the chronic liver disease population .
Toxicity
The most common adverse reactions reported in at least 3% of patients were pyrexia, abdominal pain, nausea, headache, fatigue, and peripheral edema , . Hyponatremia was also a rare serious adverse effect of this drug, seen in only 2 patients in the treatment group . Adverse reactions resulting in discontinuation of this drug have been anemia, pyrexia, and myalgia .
Atrombopag is a thrombopoietin (TPO) receptor agonist, and TPO receptor agonists have been associated with thrombotic and thromboembolic complications in patients with chronic liver disease. Portal venous thrombosis occurrence has been reported in patients with chronic liver disease who are treated with TPO receptor agonists . Patients should be monitored carefully .
Description
Avatrombopag is a medication that used for certain conditions that lead to thrombocytopenia (low platelets) such as thrombocytopenia associated with chronic liver disease in adults who are to undergo a planned medical or dental procedure.It was the third thrombopoietin receptor agonist approved for the treatment of immune thrombocytopenia and the first approved to treat periprocedural thrombocytopenia in patients with chronic liver disease (thereby providing an alternative to blood transfusions for these patients). Avatrombopag was approved for medical use in the United States in 2018, and in the European Union in 2019.
The Uses of Avatrombopag
Avatrombopag is used to treat thrombocytopenia (a low number of platelets [type of blood cell needed for blood clotting]) in people with chronic (ongoing) liver disease. It is also used to treat thrombocytopenia in people with chronic immune thrombocytopenia (ITP; an ongoing condition that may cause unusual bruising or bleeding due to an abnormally low number of platelets in the blood).
Indications
Indicated for the treatment of thrombocytopenia in adult patients with chronic liver disease who are scheduled to undergo a procedure .
Background
Avatrombopag (Doptelet), is an orally administered, small-molecule thrombopoietin receptor (c-Mpl) agonist which increases platelet number, but not platelet activation , . This decreases the need for blood transfusions .
Patients with thrombocytopenia and chronic liver disease (leading to thrombocytopenia) often require platelet transfusions before surgical procedures to decrease the risk of bleeding . Thrombocytopenia (or decreased numbers of platelets) is a common complication in patients suffering from chronic liver disease, either as an immediate result of liver disease or a consequence of interferon-based antiviral therapy .
Avatrombopag was approved by the FDA on May 21, 2018 for thrombocytopenia (low platelets) in adults with chronic liver disease who are scheduled to undergo a procedure . It is administered orally as avatrombopag maleate, its salt form .
Doptelet (Avatrombopag) is the first orally administered treatment option for patients with chronic liver disease, allowing a large population of patients to avoid a platelet transfusion before a procedure by increasing platelet counts to the optimal level of greater or equal to 50,000 per microliter .
Mechanism of action
Avatrombopag is in a class of medications called thrombopoietin (TPO) receptor agonists. It works by causing the body to produce more platelets.
Pharmacokinetics
In a study of efficacy, avatrombopag resulted in dose and exposure-dependent elevations in platelet counts in adults . The onset of the platelet count increase was noted within 3 to 5 days of the start of a 5-day treatment course, with the highest level of effect measured after 10 to 13 days. Following this, platelet counts decreased gradually, returning to near baseline values at the 35-day point .
Increased platelet activation leads to increased blood clotting, which may lead to various complications . Avatrombopag does not lead to increased platelet activation .
Metabolism
Avatrombopag is primarily metabolized by cytochrome P450 (CYP) 2C9 and CYP3A4 .
Properties of Avatrombopag
| Density | 1.440 |
| solubility | DMSO:27.33(Max Conc. mg/mL);42.07(Max Conc. mM) |
| form | A crystalline solid |
| pka | 3.04±0.70(Predicted) |
| color | White to off-white |
Safety information for Avatrombopag
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P310:Immediately call a POISON CENTER or doctor/physician. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Avatrombopag
Avatrombopag manufacturer
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde Electrolytic Iron Powder 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 4-HYDROXY BENZYL ALCOHOL 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) S-2-CHLORO PROPIONIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 3-(Hydroxymethyl)benzoate N-Boc-2-chloroethylamine 1-Bromo-2-methoxy-3-nitrobenzene N-Methyl-3-cyclopenten-1-amine 2-Bromo-3-hydroxybenzaldehyde 1H-indazole-5-carboxamideRelated products of tetrahydrofuran
![methyl 3'-amino-2'-hydroxy-[1,1'-biphenyl]-3-carboxylate](https://img.chemicalbook.in/CAS/20200611/GIF/2230800-88-9.gif)
You may like
-
 7441-43-2 98%View Details
7441-43-2 98%View Details
7441-43-2 -
 1260741-78-3 6-Bromo-3-iodo-1-methyl-1H-indazole 98%View Details
1260741-78-3 6-Bromo-3-iodo-1-methyl-1H-indazole 98%View Details
1260741-78-3 -
 (3-Benzyloxypropyl)triphenyl phosphonium 98%View Details
(3-Benzyloxypropyl)triphenyl phosphonium 98%View Details
54314-85-1 -
 4-bromo-3,5-dimethylbenzenesulfonyl chloride 1581266-79-6 98%View Details
4-bromo-3,5-dimethylbenzenesulfonyl chloride 1581266-79-6 98%View Details
1581266-79-6 -
 2490430-37-8 98%View Details
2490430-37-8 98%View Details
2490430-37-8 -
 N-(5-Amino-2-methylphenyl)acetamide 5434-30-0 98%View Details
N-(5-Amino-2-methylphenyl)acetamide 5434-30-0 98%View Details
5434-30-0 -
 124371-59-1 98%View Details
124371-59-1 98%View Details
124371-59-1 -
 53857-52-2 98%View Details
53857-52-2 98%View Details
53857-52-2