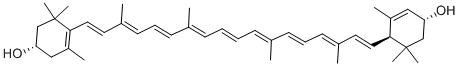
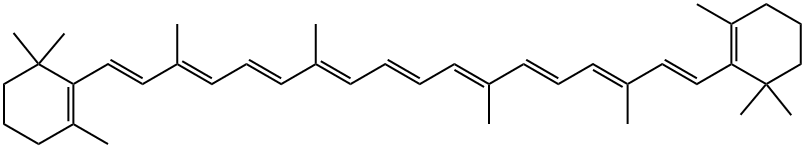
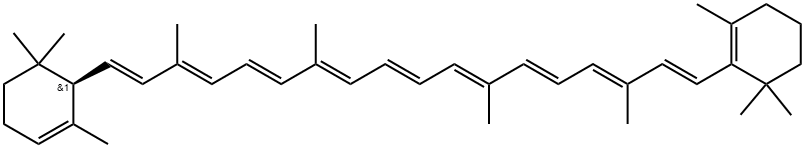
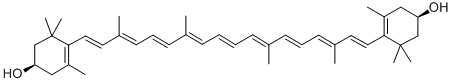
Astaxanthin
Synonym(s):Astaxanthin;trans-Astaxanthin;(3S,3′S,all-trans)-3,3′-Dihydroxy-β,β-carotene-4,4′-dione;(3S,3′S)-3,3′-Dihydroxy-β,β-carotene-4,4′-dione;(3S,3′S)-3,3′-Dihydroxy-β,β-Carotene-4,4′-dione
- CAS NO.:472-61-7
- Empirical Formula: C40H52O4
- Molecular Weight: 596.85
- MDL number: MFCD00672621
- EINECS: 207-451-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-19 17:28:17

What is Astaxanthin?
Description
Astaxanthin, a member of the carotenoid family, is part of a nutritious diet—for lobsters and shrimp. These crustaceans bind astaxanthin to the protein β-crustacyanin, which changes the shape of the astaxanthin molecules, turning them a gray-blue color. Cooking releases the molecules, returning them to their original bright red color.
Chemical properties
Drak-Purple Solid
The Uses of Astaxanthin
Astaxanthin is a carotenoid pigment found primarily in marine animals including shrimp and salmon. It is a potent lipid-soluble antioxidant.
The Uses of Astaxanthin
Carotenoid pigment found mostly in animal organisms, but also occuring in plants; thought to be the precursor of astacin. Animal studies indicate that it reduces blood glucose and ameliorates several parameters of the diabetic metabolic syndrome. It improves blood flow and vascular tone in models of hypertension.
The Uses of Astaxanthin
astaxanthin is an anti-oxidant. Molecularly, astaxanthin is similar to beta-carotene, but in clinical studies it appears to demonstrate stronger anti-oxidant properties, including an ability to inhibit lipid peroxidation and an anti-inflammatory capacity. It is used in cosmetics for its anti-oxidant properties, and for possible uV protection abilities. Astaxanthin is a naturally occurring pigment, part of the carotenoid group, and found in many foods. It is what provides salmon and certain crustaceans (e.g., shrimp, crab, lobster) with their reddish tint. Astaxanthin can also be synthetically produced.
Definition
ChEBI: Astaxanthin is a carotenone that consists of beta,beta-carotene-4,4'-dione bearing two hydroxy substituents at positions 3 and 3' (the 3S,3'S diastereomer). A carotenoid pigment found mainly in animals (crustaceans, echinoderms) but also occurring in plants. It can occur free (as a red pigment), as an ester, or as a blue, brown or green chromoprotein. It has a role as an anticoagulant, an antioxidant, a food colouring, a plant metabolite and an animal metabolite. It is a carotenone and a carotenol. It derives from a hydride of a beta-carotene.
Biotechnological Production
Commercial production processes are known in which astaxanthin production is
performed in closed photobioreactors by Cyanotech Corporation (Hawaii).
This mode of cultivation strongly improves process control, algae predation, and
production, but the fermentation costs are significantly higher. These higher
costs have to be compensated by high productivity levels and more efficient
downstream processing in order to make a competitive product.
Most of the astaxanthin available on the world market has been produced
chemically since the 1950s, for example, by DSM and BASF, and the process is
efficient and cost-effective. There are also microbial sources, for example,
X. dendrorhous (previously described as P. rhodozyma), and the alga H. pluvialis.
X. dendrorhous has been widely investigated as to its potential of astaxanthin
production for salmonids. This has resulted in a race between companies
in the 1980s and 1990s. Natural astaxanthin is currently available as a
spray-dried powder (5–10 mg astaxanthin/g), and is supplemented to fish feed to
give salmonid flesh its pink color. Another astaxanthin application is in the
nutraceutical market, as astaxanthin is regarded as a potential antioxidant.
With regard to production by algae, H. pluvialis is the only species commercially
cultivated for astaxanthin production. This green alga is able to grow under
autotrophic, heterotrophic, and mixotrophic conditions. An example of the latter is
its ability to grow in the presence of acetate and light. Astaxanthin accumulates
in response to stress in lipid globules of the cells.
General Description
all-trans-Astaxanthin is a ketocarotenoid, most commonly identified in marine and aquatic animals, including krill, wild salmon, rainbow trout, microalgae, shells of lobster, shrimp, seafood products, etc. It is biologically known as a vitamin A precursor, and exhibits strong antioxidant property, much higher compared to vitamin E and β-carotene. Its role in food and the medicinal industry is also well-defined.
Biochem/physiol Actions
Astaxanthin is a potent carotenoid antioxidant found in marine algae, red yeast and many other plant and animal sources. Animal studies indicate that it reduces blood glucose and ameliorates several parameters of the diabetic metabolic syndrome. It improves blood flow and vascular tone in models of hypertension. In vitro studies indicate that it upregulates connexin 43 and thus, may be chemopreventive against cancer.
Properties of Astaxanthin
| Melting point: | 215-216 °C |
| Boiling point: | 568.55°C (rough estimate) |
| Density | 0.9980 (rough estimate) |
| refractive index | 1.4760 (estimate) |
| storage temp. | -20°C |
| solubility | DMSO: soluble1mg/mL (warmed) |
| form | powder |
| pka | 12.33±0.70(Predicted) |
| color | , pink to very dark purple |
| Stability: | Light Sensitive |
| CAS DataBase Reference | 472-61-7 |
Safety information for Astaxanthin
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P271:Use only outdoors or in a well-ventilated area. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. P405:Store locked up. P403+P233:Store in a well-ventilated place. Keep container tightly closed. |
Computed Descriptors for Astaxanthin
| InChIKey | MQZIGYBFDRPAKN-SODZLZBXSA-N |
| SMILES | C(/C1=C(C(=O)[C@@H](O)CC1(C)C)C)=C\C(\C)=C\C=C\C(\C)=C\C=C\C=C(/C)\C=C\C=C(/C)\C=C\C1=C(C(=O)[C@@H](O)CC1(C)C)C |&1:5,37,r| |
Astaxanthin manufacturer
The Bangalore Sales Corporation
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 472-61-7 Astaxanthin 98%View Details
472-61-7 Astaxanthin 98%View Details
472-61-7 -
 472-61-7 99%View Details
472-61-7 99%View Details
472-61-7 -
 Astaxanthin 98%View Details
Astaxanthin 98%View Details
472-61-7 -
 Astaxanthin 472-61-7 98%View Details
Astaxanthin 472-61-7 98%View Details
472-61-7 -
 all-trans-Astaxanthin 98% (HPLC) CAS 472-61-7View Details
all-trans-Astaxanthin 98% (HPLC) CAS 472-61-7View Details
472-61-7 -
 all-trans-Astaxanthin CAS 472-61-7View Details
all-trans-Astaxanthin CAS 472-61-7View Details
472-61-7 -
 10% Astaxanthin Powder, For Pharmaceutical chemicals, 25 kg BagView Details
10% Astaxanthin Powder, For Pharmaceutical chemicals, 25 kg BagView Details
472-61-7 -
 Astaxanthin Powder , Packaging Size: 25kgView Details
Astaxanthin Powder , Packaging Size: 25kgView Details
472-61-7
