β-Carotene
Synonym(s):β,β-Carotene;β-Carotene;β-Carotene - CAS 7235-40-7 - Calbiochem;Provitamin A
- CAS NO.:7235-40-7
- Empirical Formula: C40H56
- Molecular Weight: 536.87
- MDL number: MFCD00001556
- EINECS: 230-636-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-27 15:38:00
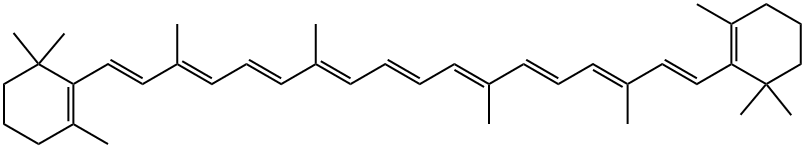
What is β-Carotene?
Absorption
After administration of beta-carotene, some of the administered dose is absorbed into the circulatory system unchanged and stored in the fat tissue. The coadministration of beta-carotene and a high-fat content diet is correlated to a better absorption of beta-carotene. The absorption is also dependent on the isomeric form of the molecule where the cis conformation seems to present a higher bioavailability. The absorption of beta-carotene is thought to be performed in 6-7 hours.
The reported AUC of beta-carotene when administered orally from 0 to 440 hours after initial administration was reported to be 26.3 mcg.h/L. The maximal concentration of beta-carotene is attained in a dual pharmacokinetic profile after 6 hours and again after 32 hours with a concentration of 0.58 micromol/L.
Toxicity
Beta-carotene is not toxic but the high and constant administration of this substance can translate into skin yellow coloration. Some reports have indicated that administration of high and periodic doses of beta-carotene are correlated to the increase in cancer incidence. This risk seems to be very elevated in the case of smokers. The registered LD50 of beta-carotene is >5000 mg/kg.
Description
beta-Carotene is widely distributed in both plant and animal kingdoms and is the most important pro-vitamin A. In plants, it is almost always found with chlorophyll.
Description
β-Carotene, one of several naturally occurring carotene isomers, is the most important of the A provitamins. It is widely distributed in the animal and plant kingdoms and is most abundant in yellow and orange fruits and vegetables such as mangoes, papayas, yams, and carrots. In biological systems, it is cleaved at the central C=C bond to form vitamin A by the enzyme β-carotene dioxygenase.
Chemical properties
Yellow to orange solid.beta-Carotene is insoluble in water, but is available in water-dispersible, oil-dispersible and oil-soluble forms. It has the activity of vitamin A.
Physical properties
β-Carotene is a tetraterpene with 11 conjugated double bonds that give the molecule an orange color. It is a carotenoid compound that is present in large quantities in the human diet and subsequently is found in all human tissues, including blood. High temperature encourages the isomerization of the double bonds, which lightens the color. Absorption (blue) and fluorescence emission (red) spectra at four excitation wavelengths from β-carotene in hexane solvent at 23 °C are shown below.
Originator
Carotaben,Hermal,W. Germany,1975
Occurrence
Beta-carotene is available naturally in fruits and vegetables. Synthetically, it may be manufactured from fungi or algae.
The Uses of β-Carotene
Beta-carotene is a known antioxidant, and antioxidants are substances that may protect your cells from free radicals, which may play a role in heart disease, cancer and other diseases. beta-carotene is a coloring agent used in margarine, cheese and pudding to produce the desired color, and is also used as an additive to yellow-orange color. beta-carotene is also a precursor to carotenoids and vitamin A It is beneficial in protecting the skin from dryness and peeling. It also slows cognitive decline and is beneficial to human health.
Background
Beta-carotene, with the molecular formula C40H56, belongs to the group of carotenoids consisting of isoprene units. The presence of long chains of conjugated double bonds donates beta-carotene with specific colors. It is the most abundant form of carotenoid and it is a precursor of the vitamin A. Beta-carotene is composed of two retinyl groups. It is an antioxidant that can be found in yellow, orange and green leafy vegetables and fruits. Under the FDA, beta-carotene is considered as a generally recognized as safe substance (GRAS).
Indications
Beta-carotene is FDA approved to be used as a nutrient supplement and to be even added in infant formula as a source of vitamin A. It is also approved to be used as a color additive for food products, drugs (with the label of "only as a color additive") and cosmetics.
It is used commonly for the reduction of photosensitivity in patients with erythropoietic protoporphyria and other photosensitivity diseases.
What are the applications of Application
β-Carotene is a terpenoid that acts as an antioxidant and provitamin
Definition
ChEBI: A cyclic carotene obtained by dimerisation of all-trans-retinol. A strongly-coloured red-orange pigment abundant in plants and fruit and the most active and important provitamin A carotenoid.
Manufacturing Process
3.6 g (0.023 mol) of 3,8-dimethyl-3,5,7-decatrien-1,9-diyne were dissolved in
50 ml of absolute ether, and to the solution was added 0.05 mol of ethereal
phenyl-lithium solution. The mixture was refluxed for 30 minutes. Then a
solution of 11 g (0.05 mol) of 4-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2-methyl-
2-buten-1-al in 100 ml of ether was added dropwise, and the reaction mixture
was boiled for 2 hours. The reaction mixture was then hydrolyzed with
aqueous ammonium acetate solution, and the ethereal layer was separated,
dried and concentrated. The residue, i.e., 1,18-di(2,6,6-trimethyl-1-
cyclohexen-1-yl)-3,7,12,16-tetramethyl-4,15-dihydroxy-2,7,9,11,16-
octadecapentaen-5,13-diyne, was a resinous product (having 1.9 active
hydrogen atoms and absorption maxima in the ultraviolet spectrum at 326
and 341 nm) which was used for the next step without any further
purification. The resin was dissolved in 200 ml of methylene chloride, 10 ml of
glacial acetic acid were added to the solution, and the mixture was cooled to -
40°C in a carbon dioxide atmosphere, while stirring. Then, 9 ml of aqueous
hydrobromic acid (60%) were added in one portion, the mixture was stirred at
-35°C for 1.5 minutes, and subsequently 200 ml of ice water were run into
the mixture. After further stirring the mixture for 2 hours at 0°C, the
methylene chloride layer was separated, washed with water and sodium
bicarbonate solution, dried with Na2SO4 andconcentrated in vacuo. The
residue, i.e., 11,12-11',12'-bisdehydro-betta-carotene, was a tough resin or a
foamy solid (having no active hydrogen atoms and possessing absorption
maxima in the ultraviolet spectrum at 334 and 408 nm). This product can be
purified by chromatography. The crude product can also be used for the next
step without any preliminary purification.
11.4 g of 11,12-11',12'-bisdehydro-β-carotene were dissolved in 100 ml of
petroleum ether (boiling range 80° to100°C), and the solution was
hydrogenated under normal conditions after the addition of 0.5 ml of quinoline
and 5 g of a lead-poisoned palladium catalyst. After the calculated amount of
hydrogen had been absorbed, the catalyst was removed by filtration and the
filtrate was extracted with dilute sulfuric acid to remove the quinoline. By
concentrating the solution in the usual manner there was obtained 11,12-
11',12'-di-cis-carotene. The product was purified by recrystallization from
benzene-alcohol. The purified product melts at 154°C; absorption maxima in
the ultraviolet spectrum at 276, 334, 338, 401 and 405 nm. The isomerization
was effected by heating the product for 10 hours at 90 to 100°C in high-boiling petroleum ether in a carbon dioxide atmosphere. The resulting and carotene melted at 180°C; ultraviolet absorption maxima at 452 and 480 nm.
Preparation of the intermediates for the above chemical synthesis are also
described in US. Patent 2,917,539. The other patents cited below describe a
fermentation route. US Patent 2,848,508 describes preparation from carrots.
brand name
BetaVit (BASF); Lucaratin (BASF); Solatene (Hoffmann-LaRoche).
Therapeutic Function
Vitamin A precursor, Sunscreen agent
General Description
beta-Carotene is an antioxidant and is one of the most important carotenoids and a source of vitamin A. It is abundantly present in fruits and vegetables which is also used as a food supplement and a colorant.
Biochem/physiol Actions
The most important of the provitamins A, β-carotene can be classified as an antioxidant due to its inhibition of radical initiated peroxidation in vitro. However, in vivo it appears to act either as an antioxidant or a prooxidant depending on cellular environment. It reduces the incidence of many cancers, but enhances lung cancer incidence in smokers.
Pharmacokinetics
Oral administration of beta-carotene increases the serum concentration of beta-carotene by 60% but it does not change the concentration found in the heart, liver or kidneys. In vitro studies in hepatocytes have shown that beta-carotene ameliorates oxidative stress, enhances antioxidant activity and decreases apoptosis.
Other than the antioxidant activities, some other actions have been correlated to beta-carotene. It is thought to have detoxifying properties, as well as to help increase resistance to inflammation and infection and increase immune response and enhance RNA production.
Side Effects
Side effects from β-Carotene include: Skin discoloration (yellowing that eventually goes away); Loose stools; Bruising; Joint pain.
Safety Profile
When heated to decomposition it emits acrid smoke and irritating fumes.
Source
The richest sources of β-Carotene are yellow, orange, and green leafy fruits and vegetables (such as carrots, spinach, lettuce, tomatoes, sweet potatoes, broccoli, cantaloupe, and winter squash). In general, the more intense the color of the fruit or vegetable, the more beta-carotene it has.
Metabolism
Beta-carotene is broken down in the mucosa of the small intestine and liver by beta-carotene dioxygenase to retinal which is a form of vitamin A. The function of this enzyme is vital as it decides if the beta-carotene is transformed to vitamin A or if it circulates in the plasma as beta-carotene. Less than a quarter of the ingested beta-carotene from root vegetables and about half of the beta-carotene from leafy green vegetables are converted to vitamin A.
Purification Methods
It forms purple prisms when crystallised from *C6H6/MeOH and red rhombs from pet ether. Its solubility in hexane is 0.1% at 0o. It is oxygen sensitive and should be stored under N2 at -20o in the dark. It gives a deep blue colour with λmax at 590nm when mixed with SbCl3 in CHCl3. UV: (*C6H6) 429infl, max at 454 and 484nm. The principal peak at 454nm has 1cm 1% 2000. [Synthesis: Surmatis & Ofner J Org Chem 26 1171 1961; Milas et al. J Am Chem Soc 72 4844 1950.] β-Carotene is also purified by column chromatography (Al2O3 activity I-II). It is dissolved in pet ether/*C6H6 (10:1), applied to the column and eluted with pet ether/EtOH; the desired fraction is evaporated and the residue is recrystallised from *C6H6/MeOH (violet-red plates). [UV: Inhoffen et al. Justus Liebigs Ann Chem 570 54, 68 1950; Review: Fleming Selected Organic Synthesis (J Wiley, Lond) pp. 70-74 1973.] Alternatively it can be purified by chromatography on a magnesia column, thin layer of Kieselguhr or magnesia. Crystallise it from CS2/MeOH, Et2O/pet ether, acetone/pet ether or toluene/MeOH. Store it in the dark, under an inert atmosphere, at -20o. Recrystallise it also from 1:1 EtOH/CHCl3. [Bobrowski & Das J Phys Chem 89 5079 1985, Johnston & Scaiano J Am Chem Soc 1 0 8 2349 1986, Strain J Biol Chem 105 523 1934, Meth Biochem Anal 4 1 1957, Beilstein 5 II 638, 5 III 2453, 5 IV 2617.]
Properties of β-Carotene
| Melting point: | 178-179 °C |
| Boiling point: | 644.94°C (rough estimate) |
| Density | 1.000 |
| vapor pressure | 0.004Pa at 25℃ |
| refractive index | 1.5630 (estimate) |
| Flash point: | 103 °C |
| storage temp. | -20°C |
| solubility | hexane: 100 μg/mL, soluble |
| form | powder |
| color | red to purple |
| Water Solubility | Soluble in hexane, dimethyl sulfoxide, benzene, chloroform, cyclohexane. Insoluble in water. |
| Sensitive | Air & Light Sensitive |
| Merck | 14,1853 |
| BRN | 1917416 |
| Stability: | Stable, but sensitive to air, heat and light. Store at -20C under nitrogen. Pyrophoric - may ignite spontaneously in air at room temperature. |
| CAS DataBase Reference | 7235-40-7(CAS DataBase Reference) |
| NIST Chemistry Reference | «beta» Carotene(7235-40-7) |
| EPA Substance Registry System | .beta.,.beta.-Carotene (7235-40-7) |
Safety information for β-Carotene
Computed Descriptors for β-Carotene
| InChIKey | OENHQHLEOONYIE-JLTXGRSLSA-N |
β-Carotene manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran






You may like
-
 BETA CAROTENE 99%View Details
BETA CAROTENE 99%View Details -
 trans beta-Carotene 98%View Details
trans beta-Carotene 98%View Details
7235-40-7 / 116-32-5 -
 β-Carotene CAS 7235-40-7View Details
β-Carotene CAS 7235-40-7View Details
7235-40-7 -
 ß-Carotene extrapure CAS 7235-40-7View Details
ß-Carotene extrapure CAS 7235-40-7View Details
7235-40-7 -
 β-Carotene CAS 7235-40-7View Details
β-Carotene CAS 7235-40-7View Details
7235-40-7 -
 Beta Carotene CAS 7235-40-7View Details
Beta Carotene CAS 7235-40-7View Details
7235-40-7 -
 beta-Carotene 93% (HPLC) CAS 7235-40-7View Details
beta-Carotene 93% (HPLC) CAS 7235-40-7View Details
7235-40-7 -
 Natural Beta Carotene, 10Kg BagView Details
Natural Beta Carotene, 10Kg BagView Details
7235-40-7
