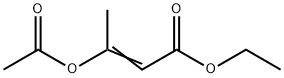
Acetyl ketene
- CAS NO.:674-82-8
- Empirical Formula: C4H4O2
- Molecular Weight: 84.07
- MDL number: MFCD00005173
- EINECS: 211-617-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-07-04 15:08:32
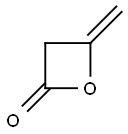
What is Acetyl ketene?
Chemical properties
Diketene [674-82-8], C4H4O2, Mr 84.04, is a colorless liquid when pure; it possesses a pungent odor and is a strong lachrymator. The human odor perception threshold is 0.019 mg/m3. Crude diketene obtained from the dimerization of ketene is often used without further purification; it is dark brown and contains up to 10 % higher ketene polymers.
The Uses of Acetyl ketene
Diketene is used as a reactant in the manufacture of paper and control of its sizing. In addition its used in the preparation of potent and selective inhibitor of histone methyltransferase EZH2 in the treatment of B-Cell lymphomas.
General Description
A colorless liquid with a disagreeable odor. Slightly less dense than water. Flash point 90°F. Irritates skin and eyes. Used to make paints and pharmaceuticals.
Air & Water Reactions
Highly flammable. Slightly soluble in water.
Reactivity Profile
DIKETENE is a very reactive dimer, Acetyl ketene may undergo a spontaneous decomposition followed by explosion or ignition [Vervalin, 1973, p.86]. In the presence of bases, amines, mineral acids or Lewis acids a violent polymerization will occur, accompanied by gas evolution [Zdenek, F. et al., Czech Pat. 156 584, 1975].
Health Hazard
TOXIC; may be fatal if inhaled, ingested or absorbed through skin. Inhalation or contact with some of these materials will irritate or burn skin and eyes. Fire will produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution.
Fire Hazard
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion and poison hazard indoors, outdoors or in sewers. May polymerize explosively when heated or involved in a fire. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.
Chemical Reactivity
Diketene is a reactive, versatile compound, which cannot be stored indefinitely, even in the presence of stabilizing agents such as sulfates, borates, or sulfur. On standing for several days or weeks, it turns yellow and then brown. Glass vessels are not recommended for storage because the basicity of glass promotes decomposition: stainless steel or aluminum is preferred. Diketene reactions proceed mainly by ring opening and are highly exothermic. Traces of base or strong acid can lead to violent, almost explosive polymerization, and due care should be exercised when handling diketene.
Solubility in organics
Diketene is miscible with acetic anhydride, with many ketones and esters (e.g., acetone, methyl acetate, ethyl acetate, butyl acetate) with diethyl ether, tetrahydrofuran, chlorinated hydrocarbons, acetonitrile, and toluene. Furthermore, contrary to most literature reports, it is slightly soluble in aliphatic hydrocarbons such as hexane (ca. 12.5 wt % at 23℃), octane, cyclohexane, and methylcyclohexane. It is very slightly soluble in water, in which it decomposes slowly.
Properties of Acetyl ketene
| Melting point: | -7.5°C |
| Boiling point: | 69-70 °C100 mm Hg(lit.) |
| Density | 1.09 g/mL at 25 °C(lit.) |
| vapor density | 2.9 (vs air) |
| vapor pressure | 7.9 mm Hg ( 20 °C) |
| refractive index | n |
| Flash point: | 95 °F |
| storage temp. | 2-8°C |
| CAS DataBase Reference | 674-82-8(CAS DataBase Reference) |
| NIST Chemistry Reference | 2-Oxetanone, 4-methylene-(674-82-8) |
| EPA Substance Registry System | Diketene (674-82-8) |
Safety information for Acetyl ketene
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H226:Flammable liquids H302:Acute toxicity,oral H331:Acute toxicity,inhalation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P403+P233:Store in a well-ventilated place. Keep container tightly closed. P403+P235:Store in a well-ventilated place. Keep cool. |
Computed Descriptors for Acetyl ketene
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran




You may like
-
 674-82-8 / 6842-10-0 Diketene 98%View Details
674-82-8 / 6842-10-0 Diketene 98%View Details
674-82-8 / 6842-10-0 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
