62-53-3
Product Name:
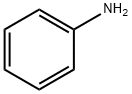
Aniline
Formula:
C6H7N
Synonyms:
Aminobenzene, Phenylamine, Benzenamine;Aniline
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Aniline appears as a yellowish to brownish oily liquid with a musty fishy odor. Melting point -6 °C; boiling point 184 °C; flash point 158 °F. Denser than water (8.5 lb / gal) and slightly soluble in water. Vapors heavier than air. Toxic by skin absorption and inhalation. Produces toxic oxides of nitrogen during combustion. Used to manufacture other chemicals, especially dyes, photographic chemicals, agricultural chemicals and others. |
|---|---|
| Color/Form | Oily liquid; colorless when freshly distilled, darkens on exposure to air and light |
| Odor | Hedonic tone; pungent |
| Taste | Burning taste |
| Boiling Point | 363 to 367 °F at 760 mmHg (EPA, 1998) |
| Melting Point | 21 °F (EPA, 1998) |
| Flash Point | 158 °F (EPA, 1998) |
| Solubility | 10 to 50 mg/mL at 73 °F (NTP, 1992) |
| Density | 1.022 at 68 °F (EPA, 1998) - Denser than water; will sink |
| Vapor Density | 3.22 (EPA, 1998) - Heavier than air; will sink (Relative to Air) |
| Vapor Pressure | 0.67 mmHg at 77 °F (EPA, 1998) |
| LogP | 0.9 |
| Henry's Law Constant | Henry's Law constant = 2.02X10-6 atm-cu m/mol at 25 °C |
| Stability/Shelf Life | Stable under recommended storage conditions. |
| Autoignition Temperature | 1139 °F (USCG, 1999) |
| Decomposition | Hazardous decomposition products formed under fire conditions - Carbon oxides, nitrogen oxides (NOx). |
| Viscosity | 4.35 cP at 20 °C; 1.62 cP at 60 °C |
| Heat of Combustion | -3392.8 KJ/mol |
| Heat of Vaporization | 55.83 kJ/mol |
| pH | pH = 8.1 (0.2 molar aq soln) |
| Surface Tension | 42.12 dynes/cm at 25 °C in contact with air; 39.41 dynes/cm at 50 °C in contact with air; 36.69 dynes/cm at 75 °C in contact with air. |
| Ionization Potential | 7.70 eV |
| Ionization Efficiency | Positive |
| Polymerization | Unless inhibited (usually methanol), aniline is readily able to polymerize. |
| Odor Threshold | Odor Threshold Low: 0.58 [mmHg] Odor Threshold High: 10.0 [mmHg] Detection odor threshold from AIHA (mean = 2.4 ppm) |
| Refractive Index | Index of refraction: 1.5863 at 20 °C/D |
| Dissociation Constants | 4.6 (at 25 °C; aniline conjugate acid) |
| Relative Evaporation Rate | < 1 (Butyl acetate = 1) |
| Kovats Retention Index | 939.2 945.6 955 955 939.1 952.6 966 967 968 968 983 995 995 946 945 946 947 950 954 947 952 958 964 963 953.3 |
| Other Experimental Properties | Easily acylated and alkylated ... easily oxidized to complex self-condensation dyes known as aniline blacks |
| Chemical Classes | Nitrogen Compounds -> Amines, Aromatic |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H317:Sensitisation, Skin H318:Serious eye damage/eye irritation H341:Germ cell mutagenicity H351:Carcinogenicity H372:Specific target organ toxicity, repeated exposure H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 93.13 g/mol |
|---|---|
| XLogP3 | 0.9 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 0 |
| Exact Mass | 93.057849228 g/mol |
| Monoisotopic Mass | 93.057849228 g/mol |
| Topological Polar Surface Area | 26 Ų |
| Heavy Atom Count | 7 |
| Formal Charge | 0 |
| Complexity | 46.1 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Aniline is a clear to slightly yellow liquid with a characteristic odor. It does not readily evaporate at room temperature. Aniline is slightly soluble in water and mixes readily with most organic solvents. Aniline is used to make a wide variety of products such as polyurethane foam, agricultural chemicals, synthetic dyes, antioxidants, stabilizers for the rubber industry, herbicides, varnishes and explosives
RELATED SUPPLIERS
Panoli Intermediates (India) Pvt., Limited. (Kutch Chemical Industries Ltd.)
1Y
product:Aniline 62-53-3 99%