Anethole trithione
- CAS NO.:532-11-6
- Empirical Formula: C10H8OS3
- Molecular Weight: 240.36
- MDL number: MFCD00129751
- EINECS: 208-528-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 13:58:55
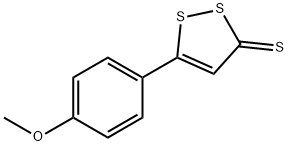
What is Anethole trithione?
Absorption
Although anethole trithione (ATT) has a high lipophilicity (log P = 3.8) and a high intestinal permeability, it has an extremely low water solubility (0.38 ug/ml). This low solubility limits ATT dissolution and bioavailability .
Regardless, after ATT was administered to twenty-two healthy Chinese volunteers, the Cmax observed was about 0.98 +/- 0.49 ng/mL and the recorded Tmax was 2.2 +/- 1.9 h .
Toxicity
Data regarding the overdosage and toxicity of anethole trithione (ATT) is not readily accessible. Nevertheless, some common side effects associated with taking ATT include softening of stool and/or discoloration of the urine to a bright yellow .
Originator
Sialor,Paladin Labs
The Uses of Anethole trithione
choleretic
Indications
The most typical uses for which anethol trithione is currently indicated for includes increasing salivary secretion in patients experiencing dry mouth or being used as an adjunctive therapy for cholecystitis, gallstone, indigestion, and acute/chronic hepatitis .
In addition, although some studies have suggested that anethol trithione also possesses a certain capacity to inhibit tumorigenesis as a potential cancer therapy medication, the specific mechanism of action for this effect remains to be elucidated with certain national cancer institutes listing the agent as 'a substance that is being studied in the treatment of cancer' .
Background
Anethole trithione (ATT) appears to have a broad range of unique functions, from increasing salivary secretion to help treat xerostomia , to demonstrating an ability to inhibit carcinogenesis by increasing the activity of electrophile detoxification enzymes , and even being used as an adjunctive therapy for cholecystitis, gallstone, indigestion, and acute/chronic hepatitis and is marketed in certain countries like France, Germany, and China .
Unfortunately, many of the specific mechanisms of action to these activities have yet to be formally elucidated, which means that while studies are ongoing, ATT itself is not necessarily formally indicated for many of these aforementioned functions at this time and is only used in limited regions around the world.
Definition
ChEBI: Anetholtrithion is a member of methoxybenzenes.
Manufacturing Process
1 mol anethol (1-methoxy-4-propenyl-benzene) and 3 mol sulfur were heated in an open vessel to temperature 190°-200°C. H2S was removed at this temperature. On cooling the reaction mixture was getting solid. The solid product was recrystallizated from acetone or ethanol to give the orange-red needles of 5-(4-methoxyphenyl)-3H-1,2-dithiole-3-thione; MP 108.5°C.
Therapeutic Function
Salivation stimulant, Choleretic, Neuroprotective
Pharmacokinetics
Anethol trithione (ATT) possesses a high lipophilicity (log P = 3.8) but an extremely low water solubility (0.38 ug/mL), which limits its dissolution and absorption . Furthermore, ATT is quickly metabolized into 4-hydroxy-anethole trithione (ATX, which demonstrates a similar pharmacological activity to ATT) by way of O-demethylation . As a consequence, the plasma concentration of ATT is usually fairly low, resulting in a limited oral bioavailability as well . Given this pharmacodynamic profile, there is continued interest and study in developing vehicles with which ATT can be administered in larger availabilities into the body .
Safety Profile
Poison by intramuscular route.Moderately toxic by ingestion and intraperitoneal routes.Experimental reproductive effects. When heated todecomposition it emits toxic fumes of SOx.
Metabolism
Anethole trithione (ATT) is metabolized rapidly into 4-hydroxy-anethole trithione via O-demethylation . This metabolite demonstrates similar pharmacological activities to its parent, ATT . It is proposed that such metabolism occurs in liver microsomes, although neither this proposal or by what specific hepatic cytochrome P450 isoform(s) are involved in such metabolism has been formally elucidated .
Properties of Anethole trithione
| Melting point: | 111° |
| Boiling point: | 353.09°C (rough estimate) |
| Density | 1.4406 (rough estimate) |
| refractive index | 1.5080 (estimate) |
| storage temp. | 2-8°C |
| solubility | soluble in Chloroform |
| form | powder to crystal |
| color | Orange-colored prisms from butyl acetate |
| Odor | very bitter taste |
| CAS DataBase Reference | 532-11-6(CAS DataBase Reference) |
| NIST Chemistry Reference | 3H-1,2-Dithiole-3-thione, 5-(4-methoxyphenyl)-(532-11-6) |
Safety information for Anethole trithione
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H332:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Anethole trithione
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran


You may like
-
 Anethole Trithione CAS 532-11-6View Details
Anethole Trithione CAS 532-11-6View Details
532-11-6 -
 Anethole trithione 99% (HPLC) CAS 532-11-6View Details
Anethole trithione 99% (HPLC) CAS 532-11-6View Details
532-11-6 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
2082-79-3 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
