Dicyclopentadiene
Synonym(s):4,7-Methano-3a,4,7,7a-tetrahydroindene;Cyclopentadiene dimer;Cyclopentadiene dimer, 4,7-Methano-3a,4,7,7a-tetrahydroindene;DCPD;Dicyclopentadiene
- CAS NO.:77-73-6
- Empirical Formula: C10H12
- Molecular Weight: 132.2
- MDL number: MFCD00078246
- EINECS: 201-052-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-10-29 10:02:07
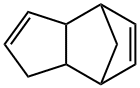
What is Dicyclopentadiene?
Description
Cyclopentadiene is a crystalline solid or a liquid (above 32℃) with a disagreeable, camphor-like odor.The odor threshold is 0.011 (detectable); 0.020 ppm (recog_x005fnizable). Molecular weight=132.22; Boilingpoint=decomposes at 172.2℃; Freezing/Melting point 532℃; Vapor pressure=1.4 mmHg at 20℃; Flash point =32℃; Autoignition temperature=503℃. HazardIdentification (based on NFPA-704 M Rating System):Health 1, Flammability 3, Reactivity 1. Explosive limits:LEL=0.8; UEL=6.3. Practically insoluble in water;solubility=0.02%.
Chemical properties
Dicyclopentadiene (DCPC) is a dimer of cyclopentadiene. First heating is used to copolymerize cyclopentadiene into dicyclopentadiene, dicyclopentadiene is separated from other light components (boiling point <45°C) by distillation, and then other required dienes, monocyclopentadiene are separated by solvent extraction. Alkene and saturated hydrocarbon components. High-purity dicyclopentadiene is a colorless crystal at room temperature. When it contains impurities, it is a light yellow oily liquid with a pungent camphor smell. It is insoluble in water and soluble in organic solvents such as alcohol and ether.
cyclopentadiene, although a stable molecule, has a strong tendency to form the more stable dimer dicyclopentadiene. This dimerisation already takes place at room temperature and its rate rapidly increases with elevated temperatures. This reaction however is reversible too; dicyclopentadiene "cracks" at temperatures above 140°C to form two cyclopentadiene molecules.
The Uses of Dicyclopentadiene
Dicyclopentadiene is used in resins, particularly unsaturated polyester resins. It plays a major role in inks, adhesives and paints. It is also used as a monomer in polymerization reactions. It is a precursor for the preparation of endo-tetrahydrodicyclopentadiene, which reacts with aluminum chloride at higher temperature to give adamantine. It undergoes a retro-Diels-Alder reaction to yield cyclopentadiene, which acts as ligand in inorganic chemistry.
The Uses of Dicyclopentadiene
Dicyclopentadiene has attracted attention as a building block for the production of modified hydrocarbon resins, which show increased reactivity in copolymerizations with drying oils and produce paint resins with improved drying rate, gloss, and hardness. dicyclopentadiene is used in the modification of tung oil, linseed oil, soybean oil, fish oil, etc., which can speed up drying and improve water resistance and alkali resistance.
Production Methods
Dicyclopentadiene is produced by recovery from hydrocarbon streams from high temperature cracked petroleum fractions. It is also a by-product of the coke oven industry. Cyclopentadiene polymerizes to dicyclopentadiene on standing.
Preparation
Dicyclopentadiene is formed by the spontaneous dimerization of cyclopentadiene by Diels-Alder reaction insolution.
What are the applications of Application
Dicyclopentadiene is mainly used in pharmaceuticals, pesticides, synthetic resins, spices, synthetic rubber and other fields. It can be used to produce adamantane, 2-Chloro-5-chloromethylpyridine, metallocene, glutaraldehyde, carbamate, epoxy resin curing agent, flame retardant, dicyclopentadiene chloride (insecticide), etc.
Definition
ChEBI: Dicyclopentadiene is a cyclic olefin.
General Description
Dicyclopentadiene appears as a liquid with an acrid odor. Flash point 90°F. The vapors are irritating to the eyes and respiratory system. Subject to polymerization if subjected to heat for prolonged periods or if contaminated. If the polymerization takes place inside a container, the container may violently rupture. Insoluble in water. Density 8.2 lb / gal. Used in paints, varnishes, as an intermediate in insecticides, as a flame retardant in plastics.
Air & Water Reactions
Highly flammable. Insoluble in water.
Reactivity Profile
Dicyclopentadiene may react vigorously with oxidizing agents. May react exothermically with reducing agents to release hydrogen gas. Can undergo exothermic polymierization reactions In the presence of various catalysts (such as acids) or initiators, if subjected to heat for prolonged periods, or if contaminated. Many undergo autoxidation upon exposure to the air to form explosive peroxides.
Health Hazard
LIQUID OR SOLID: Irritating to skin and eyes.
Fire Hazard
FLAMMABLE. Flashback along vapor trail may occur. Vapor may explode if ignited in an enclosed area.
Chemical Reactivity
Reactivity with Water No reaction; Reactivity with Common Materials: No reaction; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: May occur in presence of acids, but not hazardous; Inhibitor of Polymerization: Not pertinent.
Safety Profile
Poison by ingestion and intraperitoneal routes. Moderately toxic by inhalation. Mildly toxic by skin contact. A severe skin and moderate eye irritant. Dangerous fire hazard when exposed to heat or flame; can react with oxidizing materials. To fight fire, use alcohol foam. When heated to decomposition it emits acrid smoke and fumes.
Potential Exposure
This compound is used in the manufacture of cyclopentadiene as a pesticide intermediate; in the production of ferrocene compounds; in paints, varnishes, and resin manufacture; in production of elastomers, resin systems, and polymers.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.
Carcinogenicity
Undiluted dicyclopentadiene caused minor
irritation when applied to the skin of rabbits,
and only trace injury occurred when instilled in
the eye.
Dicyclopentadiene has a camphorlike
odor with a 100% recognition threshold of
0.02ppm; however, there may not be noticeable
irritation below 10ppm.
The 2003 ACGIH threshold limit valuetime-
weighted average (TLV-TWA) for dicyclopentadiene
is 5ppm (27mg/m3).
Storage
Color Code—Red: Flammability Hazard: Store ina flammable liquid storage area or approved cabinet awayfrom ignition sources and corrosive and reactive materials.May form peroxides in storage. Prior to working with thischemical you should be trained on its proper handling andstorage. Before entering confined space where this chemicalmay be present, check to make sure that an explosive concentration does not exist. Store in tightly closed containersin a cool, well-ventilated area away from oxidizing materials, strong acids, and strong bases. Sources of ignition, suchas smoking and open flames, are prohibited where thischemical is used, handled, or stored in a manner that couldcreate a potential fire or explosion hazard. Metal containersinvolving the transfer of=gallons or more of this chemicalshould be grounded and bonded. Drums must be equippedwith self-closing valves, pressure vacuum bungs, and flamearresters. Use only nonsparking tools and equipment, especially when opening and closing containers of thischemical.
Shipping
UN2048 Dicyclopentadiene must carry a “FLAMMABLE LIQUID” label. It falls in Hazard Class 3
Incompatibilities
Forms explosive mixture with air above flash point. Depolymerizes at boiling point and forms two molecules of cyclopentadiene; unless inhibited and maintained under inert atmosphere to prevent polymerization. Violent reaction with strong oxidizers; strong acids; strong bases. Can accumulate static electrical charges, and may cause ignition of its vapors
Waste Disposal
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed.
Properties of Dicyclopentadiene
| Melting point: | 33 °C(lit.) |
| Boiling point: | 170 °C(lit.) |
| Density | 0.986 g/mL at 25 °C(lit.) |
| vapor pressure | 3 hPa (20 °C) |
| refractive index | n |
| Flash point: | 114 °F |
| storage temp. | Store below +30°C. |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | Liquid |
| color | Clear |
| Specific Gravity | 0.968 |
| Odor | Camphor-like. |
| explosive limit | 0.8-6.3%(V) |
| Water Solubility | Immiscible with water. |
| FreezingPoint | 31.5℃ |
| Hydrolytic Sensitivity | 4: no reaction with water under neutral conditions |
| Merck | 14,2739 |
| BRN | 1904092 |
| Exposure limits | ACGIH: TWA 0.5 ppm; STEL 1 ppm NIOSH: TWA 5 ppm(30 mg/m3) |
| Dielectric constant | 2.4900000000000002 |
| Stability: | Stable at room temperature, but may form explosive peroxides if stored in contact with air. Incompatible with oxidizing agents. Decomposes on heating. Flammable. Mixtures of the vapour with air are explosive. |
| CAS DataBase Reference | 77-73-6(CAS DataBase Reference) |
| NIST Chemistry Reference | 4,7-Methano-1H-indene, 3a,4,7,7a-tetrahydro-(77-73-6) |
| EPA Substance Registry System | Dicyclopentadiene (77-73-6) |
Safety information for Dicyclopentadiene
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H225:Flammable liquids H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H330:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P273:Avoid release to the environment. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Dicyclopentadiene
| InChIKey | HECLRDQVFMWTQS-UHFFFAOYSA-N |
Dicyclopentadiene manufacturer
JSK Chemicals
Triveni Interchem Private Limited (Group Of Triveni Chemicals)
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 Dicyclopentadiene extrapure (Stabilized with BHT) CAS 77-73-6View Details
Dicyclopentadiene extrapure (Stabilized with BHT) CAS 77-73-6View Details
77-73-6 -
 Dicyclopentadiene 95% CAS 77-73-6View Details
Dicyclopentadiene 95% CAS 77-73-6View Details
77-73-6 -
 Dicylopentadiene, 95% CAS 77-73-6View Details
Dicylopentadiene, 95% CAS 77-73-6View Details
77-73-6 -
 Dicyclopentadiene CASView Details
Dicyclopentadiene CASView Details -
 Cyclopentadiene (dimer) 90% CAS 77-73-6View Details
Cyclopentadiene (dimer) 90% CAS 77-73-6View Details
77-73-6 -
 Dicyclopentadiene, CAS Number: 77-73-6View Details
Dicyclopentadiene, CAS Number: 77-73-6View Details
77-73-6 -
 Di Cyclo Pentadiene 95%View Details
Di Cyclo Pentadiene 95%View Details
77-73-6 -
 Dicyclopentadiene DcpdView Details
Dicyclopentadiene DcpdView Details
77-73-6
