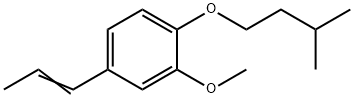
trans-Anethole
Synonym(s):trans-1-Methoxy-4-(1-propenyl)benzene;trans-Anethole;1-(4-Methoxyphenyl)-1-propene, 4-Propenylanisole, 1-Methoxy-4-propenylbenzene;4-Propenylanisole;trans-Anethole
- CAS NO.:4180-23-8
- Empirical Formula: C10H12O
- Molecular Weight: 148.2
- MDL number: MFCD00009284
- EINECS: 224-052-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-18 19:10:02
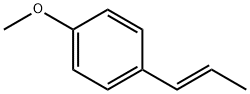
What is trans-Anethole?
Description
(E)-Anethol is a phenylpropanoid that has been found in P. anisum seed oil and has antifungal and antioxidant activity. It is active against fermentatively growing S. cerevisiae under hypoxic, but not normoxic, conditions (MIC = 100 μg/ml), and against C. parapsilosis when used at a concentration of 15% w/w. (E)-Anethol has antioxidant activity in a Trolox equivalent antioxidant capacity (TEAC) assay but does not scavenge 2,2-diphenyl-1-picrylhydrazel (DPPH) radicals in a cell-free assay.
Description
Anethole, formally 1-methoxy-4-[(1E)-prop-1-en-1-yl]benzene, is an anisole derivative that is the major constituent of the oils of anise (Pimpinella anisum), star anise (Illicium verum), and fennel (Foeniculum vulgare). Also known as “anise camphor”, it is used as a flavoring and aroma agent.
Fragrant oils have been extracted from anise and fennel plants since the Renaissance, but not until 1866 did German chemist Emil Erlenmeyer (of the eponymous flask) establish the structure of the primary substance derived from these oils. Most commercial anethole is currently derived from tree extracts such as turpentine. It is generally recognized as safe (GRAS) for use in foods, cosmetics, and pharmaceuticals.
Anethole is highly hydrophobic but soluble in ethanol, a combination that produces the “ouzo effect”. When ouzo and other anise-flavored liqueurs such as absinthe and arak are poured over ice or mixed with water, the mixtures spontaneously form highly stable emulsions. The ouzo effect is a potential mechanism for designing commercial surfactant-free emulsions without the need for expensive high-speed stabilization.
Chemical properties
trans-anethole is a clear colorless to pale yellow liquid and has a characteristic anise, sweet, spicy, warm odor and corresponding sweet taste.

Anethole (1-methoxy-4-propenyl-benzene, isoestragole) is an alkoxypropenylbenzene derivative and an important favoring component of essential oils of more than 20 plant species. Essential oils from seeds of anise (Pimpinellaanisum L.), star anise (Illicium verum Hook.f), and sweet fennel (Foeniculum vulgare Mill. var. dulce) are the main sources used for the isolation of anethole. Two isomers of anethole occur in nature: E- or trans-anethole and Z-or cis-anethole. About 90 % of natural anethole is trans-isomer. Besides separation from natural essential oils, anethole is obtained using the rectification of crude sulfate turpentine and/or the organic synthesis starting from methylchavicol or anisole and propionic anhydride. Compared to natural compound, synthetic trans-anethole is impurified with higher amounts of cis-isomer.
Occurrence
Anethole is methyl ether of oestrone and has been found in fennel, aniseed, coriander, and many other volatile oils.
The Uses of trans-Anethole
trans-Anethole is used to inhibits lung and forestomach carcinogenesis, used as carbon and energy supplement in the culture media of Pseudomonas putida strain. It is also used as used as a flavoring substance.
The Uses of trans-Anethole
expectorant, gastric stimulant, insecticide
The Uses of trans-Anethole
platelet aggregation inhibitor
The Uses of trans-Anethole
flavoring agent in food, dentifrices, etc.; in perfumery for soap, etc.; in pharmaceuticals as flavor; in photography and in embedding materials in microscopy; some perfumery uses (fennei; absinthe; Hyacinth jacinthe; detergents; magnolia). Natural occurrence: star anise
Preparation
By esterification of p-cresol with methyl alcohol and with subsequent condensation with α-cetaldehyde (Perknis); the most common method of preparation is from pine oil. By fractional distillation of the essential oils of anise, star anise, and fennel; the anise essences contain an average of 85% anethole; fennel, from 60 to 70%.
Preparation
By isomerization of estragole using alcoholic potassium hydroxide as agent (Arctander, 1969).
Definition
ChEBI: Anethole is a monomethoxybenzene that is methoxybenzene substituted by a prop-1-en-1-yl group at position 4. It has a role as a plant metabolite.
Taste threshold values
Taste characteristics at 10 ppm: sweet, anise, licorice and spicy with a lingering, sweet aftertaste.
Synthesis Reference(s)
The Journal of Organic Chemistry, 50, p. 1797, 1985 DOI: 10.1021/jo00211a002
Tetrahedron, 24, p. 2183, 1968 DOI: 10.1016/0040-4020(68)88120-7
General Description
trans-Anethole is a naturally occuring flavouring agent. It has insecticidal, larvicidal, and antimicrobial properties.
Biochem/physiol Actions
Naturally occurring phenylpropene derivative that is estrogenic at lower concentrations and cytotoxic at higher concentrations to cancer cell lines. The cytotoxicity is related to the metabolism of trans-anethole to 4-hydroxy-1-propenylbenzene.
Anticancer Research
It is one of the major constituents of essential oil of fennel and anise and belongs tothe class of phenylpropenes. It has the capacity to block both inflammation andcarcinogenesis. It is an antioxidant and also a suppressor of NF-κB activation byIκBα degradation (Aggarwal and Shishodia 2004).
Metabolism
Not Available
Metabolism
Anethole is metabolized by oxidation of the propenyl group and is excreted as anisic acid (Williams, 1959). The metabolism of trans-anethole used in the preparation of anis-flavoured alcoholic beverages was studied in the rabbit and rat after iv and oral administration. It was excreted rapidly from the animal regardless of the method of administration. After iv injection it was found concentrated in the liver, lungs and brain; after oral administration, absorption was slight and most of it remained in the stomach. Ethyl alcohol has no effect on the metabolism (Le Bourhis, 1968).
Solubility in organics
Miscible with alcohol and oils, poorly soluble in Propylene glycol and Glycerin.
Dosage
The FDA "White List" permits the use of Anise oil in concentrations up to 3500 ppm. (Anise oil contains more than 90% Anethole).
Properties of trans-Anethole
| Melting point: | 20-21 °C(lit.) |
| Boiling point: | 234-237 °C(lit.) |
| Density | 0.988 g/mL at 25 °C(lit.) |
| vapor pressure | 1.33 hPa (63 °C) |
| FEMA | 2086 | TRANS-ANETHOLE |
| refractive index | n |
| Flash point: | 195 °F |
| storage temp. | 2-8°C |
| solubility | Soluble in benzene, ethyl acetate, acetone, carbon disulfide. |
| form | Liquid After Melting |
| color | Clear colorless to pale yellow |
| PH | 7 (H2O) |
| Odor | at 10.00 % in dipropylene glycol. sweet anise licorice mimosa |
| Water Solubility | practically insoluble |
| Sensitive | Light Sensitive |
| Merck | 14,643 |
| JECFA Number | 217 |
| BRN | 774229 |
| Stability: | Light sensitive |
| CAS DataBase Reference | 4180-23-8(CAS DataBase Reference) |
| NIST Chemistry Reference | Anethole(4180-23-8) |
| EPA Substance Registry System | (E)-Anethole (4180-23-8) |
Safety information for trans-Anethole
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H317:Sensitisation, Skin H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P272:Contaminated work clothing should not be allowed out of the workplace. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P333+P313:IF SKIN irritation or rash occurs: Get medical advice/attention. |
Computed Descriptors for trans-Anethole
| InChIKey | RUVINXPYWBROJD-ONEGZZNKSA-N |
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 4180-23-8 99%View Details
4180-23-8 99%View Details
4180-23-8 -
 E-Anethole 4180-23-8 99%View Details
E-Anethole 4180-23-8 99%View Details
4180-23-8 -
 4180-23-8 E-Anethole 99%View Details
4180-23-8 E-Anethole 99%View Details
4180-23-8 -
 Anethole 99%View Details
Anethole 99%View Details -
 trans-Anethole CAS 4180-23-8View Details
trans-Anethole CAS 4180-23-8View Details
4180-23-8 -
 Anethole CAS 4180-23-8View Details
Anethole CAS 4180-23-8View Details
4180-23-8 -
 trans-Anethole CAS 4180-23-8View Details
trans-Anethole CAS 4180-23-8View Details
4180-23-8 -
 trans-Anethole CAS 4180-23-8View Details
trans-Anethole CAS 4180-23-8View Details
4180-23-8
