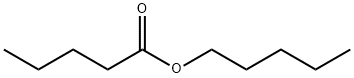
Amylamine
Synonym(s):n-Amylamine;1-Aminopentane;1-Aminopentane, n-Amylamine;Pentylamine
- CAS NO.:110-58-7
- Empirical Formula: C5H13N
- Molecular Weight: 87.16
- MDL number: MFCD00008236
- EINECS: 203-780-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02

What is Amylamine?
Chemical properties
clear colourless to very slightly yellow liquid
Chemical properties
n-Amylamine is a strong base in aqueous solutions and organic solvents that readily forms salts with acids.
Chemical properties
Colorless to yellow liquid; fishy aroma.
The Uses of Amylamine
Chemical intermediate, dyestuffs, rubber chemicals, insecticides, synthetic detergents, flotation agents, corrosion inhibitors, solvent, gasoline additive, pharmaceuticals.
The Uses of Amylamine
Amylamine is a general reagent used in functionalizing the target molecules with pentyl chain. It has also been used as a cosurfactant to increase the phase stability of the bilayer systems.
The Uses of Amylamine
1-Pentylamine is a useful reactant in organic synthesis.
Definition
ChEBI: Pentan-1-amine is a primary aliphatic amine that is n-pentane in which a hydrogen of one of the methyl groups is replaced by an amino group. A water-soluble liquid with boiling point 104℃, it is a strong irritant.
What are the applications of Application
Amylamine is A compound used in the production of dyes and emulsifiers
Production Methods
n-Amylamine is primarily produced by the amination of alkyl halides rather than using alcohol. This reaction is carried out at a temperature of 300-500°C and a pressure of 790-3550 kPa. Alternatively, n-amylamine can be produced from the reaction of amyl chlorides with ammonia. This procedure also produces small amounts of amylenes and amyl alcohol which can be removed by steam distillation (Schweizer et al 1978).
Aroma threshold values
High strength odor; fishy type; recommend smelling in a 0.10% solution or less.
General Description
A clear colorless liquid with an ammonia-like odor. Flash point 30°F. Irritates the eyes and respiratory system. Vapors are heavier than air. Produces toxic oxides of nitrogen during combustion. Used as a corrosion inhibitor, solvent, flotation agent and in the manufacture of other chemicals.
Air & Water Reactions
Highly flammable. Less dense than water and soluble in water.
Reactivity Profile
AMYLAMINES are amines. Amines are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides. Can react with oxidizing materials. [NTP 1992].
Hazard
Flammable, dangerous fire risk. Strong irritant.
Health Hazard
May cause toxic effects if inhaled or ingested/swallowed. Contact with substance may cause severe burns to skin and eyes. Fire will produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution.
Health Hazard
Direct skin contact with amylamine leads to first- and second-degree burns. Inhalation results in irritation of the mucous membranes of the nose and respiratory tract. It has been reported that in humans a concentration of 0.3 mg/1 of the inhaled n-amylamine had no irritating effect (Loit and Filou 1964).
Fire Hazard
Flammable/combustible material. May be ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.
Industrial uses
In 1976, 800 tons of n-amylamine was produced for a variety of commercial purposes. It is used in textiles, lubrication and in the manufacture of dyestuffs, emulsifying agents, anti-oxidants and desizing agents for the textile and pharmaceutical industry. It is also valuable as a corrosion inhibitor and as a base for emulsifiers which are soluble in vegetable and mineral oils.
Safety Profile
Poison by intraperitoneal route. A corrosive. A flammable liquid. When heated to decomposition it emits toxic vapors of NOx.
Metabolism
As exposure to n-amylamine is often via inhalation, several studies have investigated the uptake and distribution of amylamine by lungs. For a number of aliphatic amines their uptake correlated well with their partition coefficients (between n-octanol and pH 7 buffer) (Fowler et al 1976). The amino group, as well as the relatively lipophilic alkyl group, was required for lung specificity. It was also demonstrated using inhibitors that n-amylamine was rapidly metabolized to CO2 by monoamine oxidase and that CO2 exhalation increased with increasing chain length from C4 to C13. Another study on the pharmacokinetics of n-amylamine uptake by lung demonstrated that the distribution of n-amylamine between vascular and extravascular spaces was sensitive to arterial pH, with alkalosis favoring extravascular distribution (Effros and Chihard 1969).
The ability of n-amylamine to serve as a substrate or an inhibitor of monoamine oxidase has been addressed in a number of in vitro and in vivo studies. However, many of the results are contradictory and appear to be related to concentrationdependent phenomena. When tested in vitro, n-amylamine was reported to inhibit rat liver monoamine oxidase in a partially irreversible and noncompetitive manner (Takagi and Gomi 1966). Longer chain aliphatic amines were even more inhibitory. In contrast, at lower concentrations n-amylamine served as a substrate for monoamine oxidase. Weiner (1966) also concluded that n-amylamine was a poor substrate for monoamine oxidase isolated from rat, mouse, dog, cat, and human brains. The amine was more active towards rabbit brain monoamine oxidase. When administered intraperitoneally to rats, n-amylamine had no effect on liver monoamine oxidase activity (Valiev 1974).
Several other studies strongly suggest that amylamine is a substrate for monoamine oxidase and is metabolized by this enzyme in vivo. McEwen (1965a) purified monoamine oxidase from human plasma and found it to be most active against several simple aliphatic amines, with butylamine being the most active substrate. Further characterization indicated that high concentrations of the amine inhibited the enzyme and that the non-ionized forms of the amines are responsible for the observed competitive inhibition (McEwen 1965b). In agreement, others reported that n-amylamine was a good substrate for monoamine oxidase purified from dog serum (Ikeno et al 1978). In another in vitro study, Kurosawa (1974) demonstrated n-amylamine to be a substrate for monoamine oxidase prepared from beef or rat liver. In vivo, it was found that, in rats, the release of 14CO2 from 14C-amylamine was significantly decreased by riboflavin or iron deficiency, conditions which also decreased monoamine oxidase activity (Sourkes and Missala 1976). These studies all indicate that amylamine is metabolized by monoamine oxidase in a variety of species.
Purification Methods
Dry it by prolonged shaking with NaOH pellets, then distilling. Store it in a CO2-free atmosphere. [Beilstein 4 IV 674.]
Properties of Amylamine
| Melting point: | −55 °C(lit.) |
| Boiling point: | 104 °C(lit.) |
| Density | 0.752 g/mL at 25 °C(lit.) |
| vapor density | 3.01 (vs air) |
| vapor pressure | 120.9 hPa (47 °C) |
| FEMA | 4242 | PENTYLAMINE |
| refractive index | n |
| Flash point: | 42 °F |
| storage temp. | Flammables area |
| solubility | Soluble in alcohol, ether. |
| form | Liquid |
| pka | 10.63(at 25℃) |
| color | Clear colorless to very slightly yellow |
| Odor | at 0.10 % in propylene glycol. ammoniacal fishy |
| explosive limit | 2.2-22%(V) |
| Water Solubility | soluble |
| Sensitive | Air Sensitive |
| Merck | 14,598 |
| JECFA Number | 1585 |
| BRN | 505953 |
| Dielectric constant | 4.6(22℃) |
| CAS DataBase Reference | 110-58-7(CAS DataBase Reference) |
| NIST Chemistry Reference | 1-Pentanamine(110-58-7) |
| EPA Substance Registry System | Amylamine (110-58-7) |
Safety information for Amylamine
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H225:Flammable liquids H314:Skin corrosion/irritation H331:Acute toxicity,inhalation H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P310:Immediately call a POISON CENTER or doctor/physician. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Amylamine
Amylamine manufacturer
Sedan Speciality Chem Pvt Ltd
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 Amylamine CAS 110-58-7View Details
Amylamine CAS 110-58-7View Details
110-58-7 -
 Amylamine CAS 110-58-7View Details
Amylamine CAS 110-58-7View Details
110-58-7 -
 Amylamine, 98%+ CAS 110-58-7View Details
Amylamine, 98%+ CAS 110-58-7View Details
110-58-7 -
 Amylamine CAS 110-58-7View Details
Amylamine CAS 110-58-7View Details
110-58-7 -
 Amylamines, mixture of isomers CASView Details
Amylamines, mixture of isomers CASView Details -
 110-58-7 Amylamine 98%View Details
110-58-7 Amylamine 98%View Details
110-58-7 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1