Amoxicillin trihydrate
Synonym(s):Amoxicillin trihydrate
- CAS NO.:61336-70-7
- Empirical Formula: C16H21N3O6S
- Molecular Weight: 383.42
- MDL number: MFCD00072029
- EINECS: 999-999-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-27 15:38:00
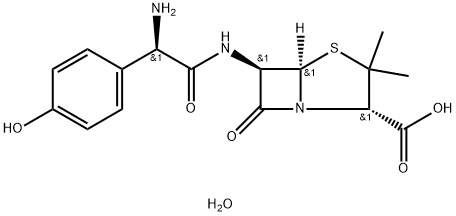
What is Amoxicillin trihydrate?
Description
Amoxicillin was synthesized by Beecham Research Laboratories in 1968. A hydroxyl group was introduced on the benzene ring of ampicillin. Amoxicillin shows about a twofold higher serum concentration than ampicillin when administered orally. It was shown by double-blind comparative studies with ampicillin that amoxicillin was as effective as ampicillin when administered at half the dose of ampicillin.
Chemical properties
White to Off-White Solid
Originator
Arnoxil,Bencard,UK,1972
The Uses of Amoxicillin trihydrate
Amoxicillin trihydratesemi-synthetic antibiotic related to Penicillin which has antibacterial poperties. It is used for treatment of a variety of bacterial infections. It is used as a pharmaceutical product as a antibiotic agent, anti-infective drug raw materials.
The Uses of Amoxicillin trihydrate
Semi-synthetic antibiotic related to Penicillin. Antibacterial.
What are the applications of Application
Amoxicillin trihydrate is a semi-synthetic antibiotic similar to penicillin
Definition
ChEBI: A hydrate that is the trihydrate form of amoxicillin; a semisynthetic antibiotic, used either alone or in combination with potassium clavulanate (under the trade name Augmentin) for treatment of a variety of bacterial infections.
Manufacturing Process
Ethyl chlorocarbonate (2.2 ml) was added to an ice cold solution of O,N-dibenzyloxycarbonyl-p-oxy-dl-α-aminophenylacetic acid (10 grams) and
triethylamine (3.85 ml) in dry acetone (193 ml). The mixture was stirred at
0°C for 5 minutes during which triethylamine hydrochloride precipitated. The
suspension was cooled to -30°C and stirred vigorously while adding as rapidly
as possible an ice cold solution of 6-aminopenicillanic acid (5.85 grams) in 3%
aqueous sodium bicarbonate (193 ml), the temperature of the mixture never
being allowed to rise above 0°C. The resulting clear solution was stirred for 30
minutes at 0°C, and then for a further 30 minutes, without external cooling,
and finally extracted with diethyl ether (3 x 200 ml) only the aqueous phase
being retained.
This aqueous solution was brought to pH 2 by the addition of hydrochloric acid
and the 6-(O,N-dibenzyloxycarbonyl-p-oxy-dl-α-
aminophenylacetamido)penicillanic acid so liberated was extracted into diethyl
ether (50 ml and 2 portions of 30 ml). The ether phase was washed with
water (3 x 5 ml) and the water washings were discarded.
Finally, the penicillin was converted to the sodium salt by shaking the ether
solution with sufficient 3% sodium bicarbonate to give a neutral aqueous
phase, separating the latter and evaporating it at low pressure and
temperature below 20°C. The product was finally dried over phosphorus
pentoxide in vacuo to give sodium 6-(O,N-dibenzyloxycarbonyl-p-oxy-dl-α-
aminophenylacetamido)-penicillanate (9.2 grams).
A suspension of palladium on calcium carbonate (36 grams of 5%) in water
(150 ml) was shaken in an atmosphere of hydrogen at room temperature and
atmospheric pressure for 1 hour. A neutral solution of sodium 6-(O,Ndibenzyloxycarbonyl-
p-oxy-dl-α-aminophenylacetamido)penicillanate (9
grams) in water (100 ml) was then added and shaking in hydrogen was
resumed for one hour. The suspension was then filtered and the collected
catalyst was washed well with water without being allowed to suck dry
between washings. The combined filtrate and washings were then brought to
pH 6.5 with dilute hydrochloric acid and evaporated to dryness at reduced
pressure and temperatures below 20°C. The product was finally dried over
phosphorus pentoxide in vacuo to give a solid (5.4 grams) containing 6-(phydroxy-
dl-α-aminophenylacetamido)penicillanic acid.
brand name
Amoxil (GlaxoSmithKline); Dispermox (Ranbaxy); Larotid (GlaxoSmithKline); Trimox (Apothecon) [Name previously used: Amoxycillin.].
Therapeutic Function
Antibacterial
Contact allergens
Amoxicillin is both a topical and a systemic sensitizer. Topical sensitization occurs in health care workers. Systemic drug reactions are frequent, such as urticaria, maculo-papular rashes, baboon syndrome, acute generalized exanthematous pustulosis, or even toxic epidermal necrosis. Cross-reactivity is common with ampicillin, and can occur with other penicillins.
Safety Profile
Moderately toxic. An experimental teratogen. Other experimental reproductive effects. When heated to decomposition it emits toxic fumes of SOx and NOx.
Properties of Amoxicillin trihydrate
| Melting point: | >200°C (dec.) |
| alpha | D20 +246° (c = 0.1) |
| refractive index | 302 ° (C=0.1, H2O) |
| storage temp. | Inert atmosphere,2-8°C |
| solubility | Slightly soluble in water, very slightly soluble in ethanol (96 per cent), practically insoluble in fatty oils. It dissolves in dilute acids and dilute solutions of alkali hydroxides. |
| form | neat |
| form | Solid |
| color | White to Off-White |
| Water Solubility | Soluble in water at 3.4mg/ml. |
| JECFA Number | 85 |
| Merck | 14,577 |
| BRN | 7507120 |
| CAS DataBase Reference | 61336-70-7(CAS DataBase Reference) |
| EPA Substance Registry System | Amoxicillin trihydrate (61336-70-7) |
Safety information for Amoxicillin trihydrate
| Signal word | Danger |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H317:Sensitisation, Skin H334:Sensitisation, respiratory |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P272:Contaminated work clothing should not be allowed out of the workplace. P280:Wear protective gloves/protective clothing/eye protection/face protection. P284:Wear respiratory protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P333+P313:IF SKIN irritation or rash occurs: Get medical advice/attention. |
Computed Descriptors for Amoxicillin trihydrate
Amoxicillin trihydrate manufacturer
Gemini Exports
Attar Global
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 61336-70-7 99%View Details
61336-70-7 99%View Details
61336-70-7 -
 Amoxicillin Trihydrate 99%View Details
Amoxicillin Trihydrate 99%View Details -
 61336-70-7 98%View Details
61336-70-7 98%View Details
61336-70-7 -
 61336-70-7 Amoxicillin Trihydrate 98%View Details
61336-70-7 Amoxicillin Trihydrate 98%View Details
61336-70-7 -
 61336-70-7 Amoxicillin Trihydrate 98%View Details
61336-70-7 Amoxicillin Trihydrate 98%View Details
61336-70-7 -
 Amoxicillin Trihydrate CAS 61336-70-7View Details
Amoxicillin Trihydrate CAS 61336-70-7View Details
61336-70-7 -
 Amoxicillin trihydrate >98% (HPLC) CAS 61336-70-7View Details
Amoxicillin trihydrate >98% (HPLC) CAS 61336-70-7View Details
61336-70-7 -
 Amoxicillin CAS 61336-70-7View Details
Amoxicillin CAS 61336-70-7View Details
61336-70-7
