Ammonium L-tartrate
Synonym(s):L -(+)-Tartaric acid diammonium salt;Diammonium tartrate
- CAS NO.:3164-29-2
- Empirical Formula: C4H12N2O6
- Molecular Weight: 184.15
- MDL number: MFCD00013073
- EINECS: 221-618-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 21:30:31
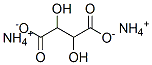
What is Ammonium L-tartrate?
Chemical properties
white crystalline powder
Chemical properties
Ammonium tartrate is a colorless crystalline or white granular solid.
The Uses of Ammonium L-tartrate
Textile industry, medicine.
The Uses of Ammonium L-tartrate
Ammonium L-(+)-tartrate finds application in the textile and pharmaceutical industries. It is also used to study protein structural analysis, biochemicals and reagents, biological buffers, chelators and proteomics. It acts as a nitrogen source in biological applications and cell culture media. Further, it is used in the study of crystal optimization research and preliminary diffraction data analysis of the Smad1 MH1 domain bonded to a palindromic SBE DNA element.
What are the applications of Application
Ammonium tartrate dibasic is used in biological applications as a nitrogen source
Definition
ChEBI: An organic salt that is the diammonium salt of L-(+)-tartaric acid.
Flammability and Explosibility
Not classified
Safety Profile
Poison by intravenous route. Moderately toxic by subcutaneous route. When heated to decomposition it emits very toxic fumes of NH3 and NOx.
Potential Exposure
Ammonium tartrate is used in the textile industry and in medicine.
Shipping
UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Strong oxidizers, especially potassium chlorate, sodium nitrite may cause violent reactions.
Waste Disposal
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed. May be buried in a chemical waste landfill in accordance with federal, state, and local statutes; or, if oxidized and neutralized, it may be sent to a municipal sewage treatment plant for biological treatment.
Properties of Ammonium L-tartrate
| Melting point: | decomposes [CRC10] |
| alpha | 32.4 º (c=1.84, H2O) |
| Boiling point: | 318.08°C (rough estimate) |
| Density | 1.601 g/mL at 25 °C(lit.) |
| vapor pressure | 0.001Pa at 20℃ |
| refractive index | 1.4800 (estimate) |
| storage temp. | room temp |
| solubility | H2O: 1 M at 20 °C, clear, colorless |
| form | crystalline |
| color | White semi-transparent |
| Specific Gravity | 1.601 |
| PH Range | 5 - 7 |
| PH | 6.0-7.5 (25℃, 1M in H2O) |
| Water Solubility | 63 g/L (15 ºC) |
| Sensitive | Hygroscopic |
| λmax | λ: 260 nm Amax: 0.052 λ: 280 nm Amax: 0.039 |
| BRN | 6120352 |
| Stability: | Stable. Incompatible with strong acids. |
| CAS DataBase Reference | 3164-29-2(CAS DataBase Reference) |
| NIST Chemistry Reference | Ammonium tartrate(3164-29-2) |
| EPA Substance Registry System | Diammonium tartrate (3164-29-2) |
Safety information for Ammonium L-tartrate
| GHS Hazard Statements |
H402:Hazardous to the aquatic environment, acute hazard H412:Hazardous to the aquatic environment, long-term hazard |
Computed Descriptors for Ammonium L-tartrate
Ammonium L-tartrate manufacturer
JSK Chemicals
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran




You may like
-
 AMMONIUM TARTRATE 99%View Details
AMMONIUM TARTRATE 99%View Details -
 Ammonium-L-tartrate CAS 3164-29-2View Details
Ammonium-L-tartrate CAS 3164-29-2View Details
3164-29-2 -
 Ammonium L-(+)-tartrate CAS 3164-29-2View Details
Ammonium L-(+)-tartrate CAS 3164-29-2View Details
3164-29-2 -
 Ammonium-L-tartrate CAS 3164-29-2View Details
Ammonium-L-tartrate CAS 3164-29-2View Details
3164-29-2 -
 Ammonium tartrate, 98% CAS 3164-29-2View Details
Ammonium tartrate, 98% CAS 3164-29-2View Details
3164-29-2 -
 Ammonium Tartrate Dibasic pure CAS 3164-29-2View Details
Ammonium Tartrate Dibasic pure CAS 3164-29-2View Details
3164-29-2 -
 Ammonium Tartrate Dibasic extrapure AR CAS 3164-29-2View Details
Ammonium Tartrate Dibasic extrapure AR CAS 3164-29-2View Details
3164-29-2 -
 Ammonium tartrate, GR 99% CAS 3164-29-2View Details
Ammonium tartrate, GR 99% CAS 3164-29-2View Details
3164-29-2
