Ammonium hexafluorosilicate
Synonym(s):Cryptohalite
- CAS NO.:16919-19-0
- Empirical Formula: F6H8N2Si
- Molecular Weight: 178.15
- MDL number: MFCD00010887
- EINECS: 240-968-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02

What is Ammonium hexafluorosilicate?
Chemical properties
white slightly adhering crystalline powder
Chemical properties
Ammonium hexafluorosilicate is a white crystalline powder. Odorless.
Physical properties
d 2.01 g cm?3; decomposes prior to melting; odorless.
The Uses of Ammonium hexafluorosilicate
Ammonium hexafluorosilicate is used as a disinfectant and analytical reagent. It finds application in etching glass, metal casting, and electroplating. It is also employed as a wood preservative agent and mothproofing agent in textiles. It plays a useful application in soldering flux. It is also used to arrest caries treatment without discoloration on demineralized primary tooth enamel.
The Uses of Ammonium hexafluorosilicate
In pesticides; in soldering flux; etching glass.
General Description
A white crystalline solid. Noncombustible. Corrodes aluminum. Used as a disinfectant, in etching glass, metal casting, and electroplating.
Air & Water Reactions
Water soluble.
Reactivity Profile
Salts, basic, such as Ammonium hexafluorosilicate, are generally soluble in water. The resulting solutions contain moderate concentrations of hydroxide ions and have pH's greater than 7.0. They react as bases to neutralize acids. These neutralizations generate heat, but less or far less than is generated by neutralization of the bases in reactivity group 10 (Bases) and the neutralization of amines. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible.
Hazard
Very toxic; fatal.
Health Hazard
Inhalation of dust can cause pulmonary irritation and can be fatal in some cases. Ingestion may be fatal. Contact with dust causes irritation of eyes and irritation or ulceration of skin.
Fire Hazard
Special Hazards of Combustion Products: Toxic and irritating hydrogen fluoride, silicon tetrafluoride, and oxides of nitrogen may form in fires.
Flammability and Explosibility
Not classified
Potential Exposure
This material is used as a pesticide and miticide, wood preservative, soldering flux; light metal casting; and in the etching of glass.
Shipping
UN2856 Fluorosilicates, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required.
Purification Methods
Crystallise the salt from water (2mL/g). After 3 recrystallisations, the Technical grade salt has Li, Na, K and Fe at 0.3, 0.2, 0.1 and 1.0 ppm respectively.
Incompatibilities
Aqueous solution is highly corrosive. Contact with acids reacts to form hydrogen fluoride, which is a highly corrosive and forms a toxic gas. Corrosive to aluminum. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Ammonium fluorosilicate reacts with water to form hydrofluoric acid, a source of fluoride ions. Unlike other halide ions, the fluoride ion is highly reactive, acting as a weak base and participating in some unique reactions. In particular, fluorides react strongly with compounds containing calcium, magnesium, or silicon ions, which means that solutions containing soluble fluorides are corrosive to both living tissue and glass. Hydrofluoric acid can cause severe chemical burns and is one of the few materials that can etch glass. It is also a toxic gas in its anhydrous form.
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. Incineration.
Properties of Ammonium hexafluorosilicate
| Melting point: | °Cd ec.) |
| Density | 2.01 g/mL at 25 °C(lit.) |
| vapor pressure | 9.99Pa at 20℃ |
| solubility | water solubility 18 g/100 g water. |
| form | Crystalline Powder and Chunks |
| color | White |
| Specific Gravity | 2.011 |
| Water Solubility | Slightly soluble in water. Insoluble in alcohol and acetone. |
| Hydrolytic Sensitivity | 0: forms stable aqueous solutions |
| Merck | 14,527 |
| Exposure limits | ACGIH: TWA 2.5 mg/m3 NIOSH: IDLH 250 mg/m3; TWA 2.5 mg/m3 |
| CAS DataBase Reference | 16919-19-0(CAS DataBase Reference) |
| EPA Substance Registry System | Ammonium silicofluoride (16919-19-0) |
Safety information for Ammonium hexafluorosilicate
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for Ammonium hexafluorosilicate
New Products
2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-Pyridineacetonitrile, α-hydroxy- 3-Iodophenylacetic acid 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Strontium Carbonate, 98% Wang resin Sodium hydrogenphosphate, anhydrous 2-Bromo-3-methoxyaniline hydrochloride, 95% (Custom work) 1-Bromo-4-chlorobenzene, 99% Benzocaine, 98% (R)-2-Methylpyrolidine-2-carboxylic acid (De Mepro) Ramipril Sacubitril- Valsartan Boc-his(trt)-OH Fmoc-L-Glu-OtBu Boc-L-Tyr(tBu)-OH Semi carbazide Hydrochloride 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 2-Chloromethyl-4-methyl-quinazoline Trans-4-Aminocyclohexanol [4tac] 2-[1-(Mercaptomethyl)Cyclopropyl]Acetic AcidRelated products of tetrahydrofuran
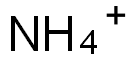
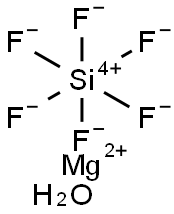

You may like
-
 AMMONIUM SILICO FLUORIDE 99%View Details
AMMONIUM SILICO FLUORIDE 99%View Details -
 16919-19-0 98%View Details
16919-19-0 98%View Details
16919-19-0 -
 Ammonium hexafluorosilicate CAS 16919-19-0View Details
Ammonium hexafluorosilicate CAS 16919-19-0View Details
16919-19-0 -
 Ammonium hexafluorosilicate CAS 16919-19-0View Details
Ammonium hexafluorosilicate CAS 16919-19-0View Details
16919-19-0 -
 Ammonium hexafluorosilicate CAS 16919-19-0View Details
Ammonium hexafluorosilicate CAS 16919-19-0View Details
16919-19-0 -
 Ammonium hexafluorosilicate, 98% CAS 16919-19-0View Details
Ammonium hexafluorosilicate, 98% CAS 16919-19-0View Details
16919-19-0 -
 15761-38-3 N-Boc-L-Alanine >98%View Details
15761-38-3 N-Boc-L-Alanine >98%View Details
15761-38-3 -
 6485-34-3 Saccharin Calcium >98%View Details
6485-34-3 Saccharin Calcium >98%View Details
6485-34-3
