Ammonium formate
Synonym(s):Formic acid ammonium salt
- CAS NO.:540-69-2
- Empirical Formula: CH5NO2
- Molecular Weight: 63.06
- MDL number: MFCD00013103
- EINECS: 208-753-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-22 14:18:24
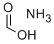
What is Ammonium formate?
Description
Ammonium formate, NH4HCO2, is the ammonium salt of formic acid. It is a colorless, hygroscopic, crystalline solid.
Description
Ammonium formate (NH4HCO2) can effectively function as a hydrogen transfer agent in the presence of Pd/C for the reduction of alkenes.
Chemical properties
colourless crystals
Physical properties
White monoclinic deliquescent crystals or granules; density 1.280 g/cm3; melts at 116°C; highly soluble in water (102 g/100 g at 0°C), solubility rapidly increasing with temperature (i.e., 531 g/100 g at 80°C); soluble in liquid ammonia, alcohol and ether.
The Uses of Ammonium formate
Ammonium formate is widely used in various organic reactions like Leuckart reaction which involves the reductive amination of aldehydes and ketones. It serves as a buffer in high performance liquid chromatography (HPLC) and liquid chromatography-mass spectrometry (LC/MS). It finds application in palladium on carbon (Pd/C) reduction of functional groups. For example, reduction of alkenes to alkanes and formaldehyde to methanol. It is also used to prepare formic acid insitu as well as used to store formic acid by making it as an ammonium salt.
The Uses of Ammonium formate

A 250 mL flask was charged with the SM (3.7 g, 19 mmol), ammonium formate (14 g, 190 mmol), and Pd/C (8.6%, 0.32 g) in MeOH (150 mL). After all the ammonium formate was dissolved, the reaction flask was evacuated and flushed with N2. The reaction mixture was stirred under inert atmosphere (N2) at RT overnight. The mixture was filtered through celite, concentrated in vacuo, diluted with 2M NaOH (ca pH 10), and extracted with EtOAc. The combined organics were washed with brine, dried (Na2SO4), and concentrated to provide the crude product which was taken forward without further purification. [3.1 g, 83%, 85:15 α:β]
The Uses of Ammonium formate
In chemical analysis, especially to ppt base metals from salts of the "noble" metals.
The Uses of Ammonium formate
Pure ammonium formate decomposes into formamide and water when heated, and this is its primary use in industry. Formic acid can also be obtained by reacting ammonium formate with a dilute acid, and since ammonium formate is also produced from formic acid, it can serve as a way of storing formic acid.
Ammonium formate can also be used in palladium on carbon (Pd / C) reduction of functional groups. In the presence of Pd / C, ammonium formate decomposes to hydrogen, carbon dioxide, and ammonia.
Ammonium formate can be used for reductive amination of aldehydes and ketones (Leuckart reaction)
Ammonium formate can be used as a buffer in high performance liquid chromatography (HPLC), and is suitable for use with liquid chromatography/mass spectrometry (LC/MS). .
What are the applications of Application
Ammonium formate is a buffer component for glycosaminoglycan purification
What are the applications of Application
Ammonium formate solution is 10 M, a buffer component for purification of glycosaminoglycans
Definition
ChEBI: The ammonium salt of formic acid.
Reactions
When heated, ammonium formate eliminates water, forming formamide. Upon further heating it forms to HCN and H2O. A side reaction of this is the decomposition of formamide to CO and NH3.
General Description
White solid with a weak odor of ammonia. Sinks and mixes slowly with water.
Air & Water Reactions
Water soluble.
Reactivity Profile
Salts, basic, such as Ammonium formate, are generally soluble in water. The resulting solutions contain moderate concentrations of hydroxide ions and have pH's greater than 7.0. They react as bases to neutralize acids. These neutralizations generate heat, but less or far less than is generated by neutralization of the bases in reactivity group 10 (Bases) and the neutralization of amines. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible.
Health Hazard
Inhalation causes irritation of nose and throat. Ingestion irritates mouth and stomach. Contact with eyes or skin causes irritation.
Fire Hazard
Special Hazards of Combustion Products: Toxic and irritating ammonia and formic acid gases may form in fire.
Flammability and Explosibility
Not classified
Safety Profile
Moderately toxic by ingestion andintravenous routes. When heated to decomposition itemits toxic fumes of NOx and NH3.
Purification Methods
Heat the solid in NH3 vapour and dry it in a vacuum till the NH3 odour is faint (note that it can evaporate completely in a vacuum). Recrystallise it from absolute EtOH and then keep it in a desiccator over 99% H2SO4 in vacuo. It is very hygroscopic. It exists in two forms, stable needles and less stable plates. It also forms acid salts, i.e. HCO2NH4.3HCO2H and HCO2NH4.HCO2H. [Kensall & Adler J Am Chem Soc 43 1473 1921, Beilstein 2 IV 18.]
Properties of Ammonium formate
| Melting point: | 119-121 °C (lit.) |
| Boiling point: | 103.28°C (rough estimate) |
| Density | 1.26 g/mL at 25 °C (lit.) |
| vapor pressure | 0.033Pa at 25℃ |
| refractive index | 1.4164 (estimate) |
| Flash point: | 104℃ |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | H2O: 10 M at 20 °C, clear, colorless |
| form | Solid |
| appearance | Colorless solid |
| color | White |
| Odor | Slight formic acid odour |
| PH | 6.53(1 mM solution);6.5(10 mM solution);6.49(100 mM solution);6.45(1000 mM solution) |
| PH Range | 6 - 7 |
| Water Solubility | soluble |
| Sensitive | Hygroscopic |
| λmax | λ: 260 nm Amax: 0.05 λ: 280 nm Amax: 0.04 |
| Merck | 14,523 |
| BRN | 3625095 |
| Stability: | Stable. Hygroscopic. Incompatible with strong acids, strong oxidizing agents. |
| CAS DataBase Reference | 540-69-2(CAS DataBase Reference) |
| EPA Substance Registry System | Ammonium formate (540-69-2) |
Safety information for Ammonium formate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for Ammonium formate
| InChIKey | VZTDIZULWFCMLS-UHFFFAOYSA-N |
Ammonium formate manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 AMMONIUM FORMATE 99%View Details
AMMONIUM FORMATE 99%View Details -
 Ammonium formate 99%View Details
Ammonium formate 99%View Details -
 Ammonium formate 98%View Details
Ammonium formate 98%View Details -
 Ammonium formate CAS 540-69-2View Details
Ammonium formate CAS 540-69-2View Details
540-69-2 -
 Ammonium formate CAS 540-69-2View Details
Ammonium formate CAS 540-69-2View Details
540-69-2 -
 Ammonium Formate ChemicalView Details
Ammonium Formate ChemicalView Details
540-69-2 -
 Ammonium Formate Powder, Technical Grade, 99%View Details
Ammonium Formate Powder, Technical Grade, 99%View Details
540-69-2 -
 Ammonium Formate, AR GradeView Details
Ammonium Formate, AR GradeView Details
540-69-2
