Ammonium ferric citrate
Synonym(s):Listeria Selective Supplement;Ammonium ferric citrate;Ammonium iron(III) citrate;Ferric ammonium citrate;FRASER Listeria Ammonium iron(III) Supplement
- CAS NO.:1185-57-5
- Empirical Formula: C6H11FeNO7
- Molecular Weight: 265
- MDL number: MFCD00013099
- EINECS: 214-686-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:50:42
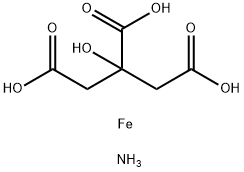
What is Ammonium ferric citrate?
The Uses of Ammonium ferric citrate
Ferric Ammonium Citrate serves as an iron supplement, produced by reacting ferric hydroxide with citric acid and ammonium hydroxide, then evaporating and drying. It is available in two forms: brown with 16.5–18.5% iron and green with 14.5–16% iron. It is used as a hematinic and in blueprints and photography. The green form, known as Iron Ammonium Citrate, also functions as an anticaking agent in salt.
Potential Exposure
Ferric ammonium citrate is used in blueprinting, photography, medical treatment; and as an animal food additive.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit. Thesymptoms of metal fume fever may be delayed for 4-12 hfollowing exposure: it may last less than 36 h.Note to physician: In case of fume inhalation, treat pneumonitis. Give prednisone or other corticosteroid orally toreduce tissue response to fume. Positive-pressure ventilationmay be necessary. Treat metal fume fever with bed rest,analgesics, and antipyretics.Note to physician: For severe poisoning do not use BAL[British Anti-Lewisite, dimercaprol, dithiopropanol(C3H8OS2)] as it is contraindicated or ineffective in poisoning from iron.
Description
Ferric ammonium citrate is a yellowish brown to red solid with a faint odor of ammonia. It is soluble in water. The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. It is used in medicine, in making blueprints, and as a feed additive.
Storage
Color Code—Green: General storage may be used.Prior to working with this chemical you should be trainedon its proper handling and storage. Store in tightly closedcontainers in a cool, well-ventilated area away from light.Sources of ignition, such as smoking and open flames, areprohibited where ferric ammonium citrate is used, handled,or stored in a manner that could create a potential fire orexplosion hazard.
Shipping
UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.
Incompatibilities
Compounds of the carboxyl group react with all bases, both inorganic and organic (i.e., amines) releasing substantial heat, water and a salt that may be harmful. Incompatible with arsenic compounds (releases hydrogen cyanide gas), diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides (releasing heat, toxic and possibly flammable gases), thiosulfates and dithionites (releasing hydrogen sulfate and oxides of sulfur).
Properties of Ammonium ferric citrate
| Melting point: | >193°C (dec.) |
| Boiling point: | 498℃[at 101 325 Pa] |
| Density | 1.06[at 20℃] |
| vapor pressure | 0Pa at 25℃ |
| storage temp. | Store at RT. |
| solubility | 1200g/l |
| form | powder |
| color | Green |
| Odor | Odorless to slight ammonia odor |
| PH | 6-8 (100g/l, H2O, 20℃) |
| Water Solubility | 1200 g/L (20 ºC) |
| Sensitive | Hygroscopic |
| Merck | 14,4017 |
| Exposure limits | ACGIH: TWA 1 mg/m3 NIOSH: TWA 1 mg/m3 |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 1185-57-5(CAS DataBase Reference) |
| EPA Substance Registry System | Ferric ammonium citrate (1185-57-5) |
Safety information for Ammonium ferric citrate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Ammonium ferric citrate
Ammonium ferric citrate manufacturer
Shanpar Industries Private Limited
Attar Global
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
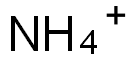
You may like
-
 Ferric Ammonium Citrate 99%View Details
Ferric Ammonium Citrate 99%View Details -
 Ferric ammonium citrate 99%View Details
Ferric ammonium citrate 99%View Details -
 Ammonium iron(III) citrate 99%View Details
Ammonium iron(III) citrate 99%View Details -
 Ammonium iron(III) citrate 98%View Details
Ammonium iron(III) citrate 98%View Details -
 Ammonium Ferric Citrate (SQ) CAS 1185-57-5View Details
Ammonium Ferric Citrate (SQ) CAS 1185-57-5View Details
1185-57-5 -
 Powder Ferric Ammonium Citrate Brown, CAS No: 1185-57-5View Details
Powder Ferric Ammonium Citrate Brown, CAS No: 1185-57-5View Details
1185-57-5 -
 Ferric Ammonium Citrate (Green/Brown)/ IP/BP/USP/BPC/FCCView Details
Ferric Ammonium Citrate (Green/Brown)/ IP/BP/USP/BPC/FCCView Details
1185-57-5 -
 FERRIC AMMONIUM CITRATEView Details
FERRIC AMMONIUM CITRATEView Details
1185-57-5
