1185-57-5
Product Name:
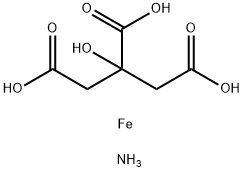
Ammonium ferric citrate
Formula:
C6H11FeNO7
Synonyms:
Listeria Selective Supplement;Ammonium ferric citrate;Ammonium iron(III) citrate;Ferric ammonium citrate;FRASER Listeria Ammonium iron(III) Supplement
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Ferric ammonium citrate is a yellowish brown to red solid with a faint odor of ammonia. It is soluble in water. The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. It is used in medicine, in making blueprints, and as a feed additive. |
|---|---|
| Density | 1.8 at 68 °F (USCG, 1999) - Denser than water; will sink |
| Decomposition | When heated to decomposition it emits acrid smoke and irritating fumes. |
| Other Experimental Properties | Brown form: reddish-brown granules, garnet-red transparent scales, or brownish-yellow powder. Very deliquescent. Odorless or slight ammonia odor; saline, ferruginous taste. Ammonium ferric citrate, brown form reduced to ferrous salt by light. Brown hydrated form is extremely soluble in water and practically insoluble in alcohol. /Hydrated ferric ammonium citrate/ |
| Chemical Classes | Metals -> Metals, Inorganic Compounds |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 261.98 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 2 |
| Exact Mass | 261.965012 g/mol |
| Monoisotopic Mass | 261.965012 g/mol |
| Topological Polar Surface Area | 142 Ų |
| Heavy Atom Count | 15 |
| Formal Charge | 0 |
| Complexity | 211 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 3 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Ammonium ferric citrate, also known as Ferric Ammonium Citrate, Ammonium Iron (III) Citrate, and Iron ammonium citrate, is a yellowish brown to reddish solid with a faint ammonia odour. It is soluble in water. It is widely used in chemical, material, pharmaceutical and food industries. It is used as a source of iron for the synthesis of iron oxide nanoparticles and for the treatment of iron deficiency anaemia; a reducer of low-activity metal salts, such as gold and silver; an iron supplement in food and a contrast agent in medical tests.
RELATED SUPPLIERS
Shanpar Industries Private Limited
Vadodara
product:Ferric Ammonium Citrate (Green/Brown)/ IP/BP/USP/BPC/FCC
