Aminopyrine
Synonym(s):4-Dimethylamino-2,3-dimethyl-1-phenyl-3-pyrazolin-5-one;Aminopyrine
- CAS NO.:58-15-1
- Empirical Formula: C13H17N3O
- Molecular Weight: 231.29
- MDL number: MFCD00003142
- EINECS: 200-365-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
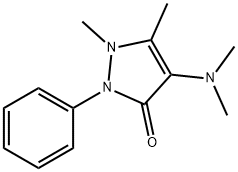
What is Aminopyrine?
Toxicity
Can cause life-threatening agranulocytosis.
Chemical properties
White leaf-like crystals or crystalline powder. Odorless, slightly bitter taste. It is stable in the air, but will deteriorate in sunlight. When there is moisture, it is easy to chemically react with weak oxidants. Soluble in alcohol, chloroform, benzene and ether, soluble in water. The solubility in water increases with the addition of sodium benzoate. Aqueous solutions are weakly alkaline to litmus. The melting point is 107-109°C.
The Uses of Aminopyrine
Aminopyrine is used as an antipyretic and analgesic drug. It belongs to the pyrazolone derivatives having a most toxic and most dangerous analgesic effect and it is a non-narcotic drug. Due to strong adverse effects, its single medicine preparation is gradually replaced by compound preparation.
What are the applications of Application
Oxidation by Fe(VI) has potential to remove N-nitrosodiumethylamine precursors, including 4-dimethylaminoantipyrine, from drinking water. 4-Dimethylaminoantipyrine is itself an effective scavenger of reactive nitrogen compounds nitric oxide and peroxynitrite, important in the inflammatory response.
Background
Aminophenazone is a pyrazolone with analgesic, anti-inflammatory, and antipyretic properties that carries a risk of agranulocytosis. In biomedical applications, radiolabelled (13C-labeled) aminophenazone has been used in breath tests to measure the cytochrome P-450 metabolic activity in liver function tests. The FDA suspended the use of aminophenazone due to its association with agranulocytosis, a life-threatening side effect.
Indications
Formerly widely used as an antipyretic and analgesic in rheumatism, neuritis, and common colds. Currently used to measure total body water.
Definition
ChEBI: Aminopyrine is a pyrazolone that is 1,2-dihydro-3H-pyrazol-3-one substituted by a dimethylamino group at position 4, methyl groups at positions 1 and 5 and a phenyl group at position 2. It exhibits analgesic, anti-inflammatory, and antipyretic properties. It has a role as a non-steroidal anti-inflammatory drug, a non-narcotic analgesic, an antipyretic, an environmental contaminant and a xenobiotic. It is a tertiary amino compound and a pyrazolone.
Preparation
Aminopyrine is synthesized from 4-Aminoantipyrine by catalytic hydrogenation (alkylation). Water is firstly added to the dissolving tank, heated to 50-60°C, put into 4-Aminoantipyrine under stirring, and poured into the hydrogenation tank after complete dissolving. Wash the dissolving tank with water, mix it with the nickel catalyst, and then add it to the hydrogenation tank. The hydrogenation tank was evacuated, then the vacuum valve was closed, hydrogen was introduced, and stirring was started. When the pressure rose to 0.245MPa, the hydrogen was stopped. Add formaldehyde and continue to feed hydrogen. The speed of adding formaldehyde is based on the standard of 1.8-2 cubic meters of hydrogen absorption per 5L of formaldehyde, and the reaction temperature is controlled at 60-85°C. After passing the test, filter by pressure, cool the filtrate to below 25°C, and separate out crystals, which are then filtered to obtain the crude aminopyrine. The crude aminopyrine, ethanol, and activated carbon were heated to 75-80°C, stirred for 1 hour, and filtered under pressure. The filtrate was cooled to 10°C, crystals were precipitated, filtered, washed with ethanol, and air-dried to obtain aminopyrine.
brand name
Adexogan;Agevis;Algimicin anttitermico;Amidazopen;Amidozen;Anoixal;Antigripina;Areumal;Axiston;Balbion;Barsedan;Baukal suppositories;Bayer 1387 p;Bronchisan;Brufaneuseol;Butapyrine;Capsyka dr knapf;Capysal;Chinopyrin;Cibalgin;Ciclazon;Clinit;Coffan;Compral;Cusayth;Demolpas;Dentigoa;Depiral c;Dexa escopyrin;Dha 51;Dialpyrin;Digisab;Dimametten;Dimopyrin;Diprin;Dolo-attirin;Dolo-optineural;Dolorphen;Dolovosano;Donobin;Duerin;Dysmensan;Escopyrinus;Espasnatex;Eufibran;Euprogan;Febren;Febrosolvin;Fenodone;Fever;Flivalgin;Flumil;Framidone;Ftalazon;Funapon;Galenopyrin;Glucopirina;Glucopitina;Helvagit-f;Hemicraneal;Hisense-p;Influnal depot;Inst;Irgapyrine;Isoftal;Kalmine;Katareuma;Lagaflex;Latepyrine;Lauroanginol;Manslu;Medispanmin;Meloka;Neuro-demoplast;Nifedon;Nikartrone;Nostress;Optineural(analgesic);Optipax;Osadrine;Osmotipax;P.s.b.p.;Piracodid;Piradenil;Piradol;Pirasco;Piraseptolo.
World Health Organization (WHO)
Aminophenazone, a pyrazolone derivative, has been used as an antiinflammatory and analgesic agent for over a century. Its use has been associated with cases of bone marrow depression and agranulocytosis and more recently it has been claimed to have a carcinogenic potential. Products containing aminophenazone have been formally withdrawn in many countries and marketing has been voluntarily suspended in others. Elsewhere, however, proprietary preparations containing this ingredient may remain available. (Reference: (WHODI) WHO Drug Information, 3, 9, 1977)
General Description
Small colorless crystals or white crystalline powder. Aqueous solution slightly alkaline to litmus. pH (5% water solution) 7.5-9. Odorless. Slightly bitter taste.
Air & Water Reactions
Water soluble.
Reactivity Profile
Aminophenazone is sensitive to exposure to light. Aminophenazone is readily attacked by mild oxidizing agents in the presence of water. Aminophenazone is incompatible with acacia, apomorphine, aspirin, chloral hydrate, iodine and tannic acid.
Fire Hazard
Flash point data for Aminophenazone are not available; however, Aminophenazone is probably combustible.
Biochem/physiol Actions
Antipyrine was found to be nonmutagenic when screened against Salmonella typhimurium tester strains TA100, TA98, TA97, TA102 and TA104.
Pharmacokinetics
Aminophenazone exhibits analgesic, anti-inflammatory, and antipyretic properties.
Safety Profile
Human poison by unspecified route. Experimental poison by ingestion, subcutaneous, intramuscular,intravenous, and intraperitoneal routes. Moderately toxic by parenteral route. Experimental teratogenic and reproductive effects. Questionable carcinogen when mixed with NaNO2 (1:l). Mutation data reported. Can cause bone marrow depression resulting in leucopenia. Has been implicated in development of aplastic anemia. A tranquilizer. When heated to decomposition it emits toxic fumes of NOx.
Metabolism
Metabolism
Aminopyrine is very slowly metabolized by normal neonate. In older infants, a higher amount of exhaled 13-CO2 is observed. In chicken aminopyrine-N-demethylase inhibits the activities of some important P-450 enzymes. It is mostly metabolized through N-demethylation to form monomethyl-4-antipyrine and formaldehyde, which can be measured by colorimetry Nash. Aminopyrine Ndemethylase is almost equal to isoform CYP-3A4 and closely linked to the methylation reaction of drugs.
why Aminopyrine Banned?
Purification Methods
It crystallises from pet ether, sublimes between 80o and 90o, and forms metal complexes. [Beilstein 25 H 452, 25 III/IV 3555.]
Properties of Aminopyrine
| Melting point: | 107-109 °C(lit.) |
| Boiling point: | 373.38°C (rough estimate) |
| Density | 1.0744 (rough estimate) |
| refractive index | 1.6140 (estimate) |
| storage temp. | Refrigerator |
| solubility | DMF: 5 mg/ml; DMSO: 5 mg/ml; Ethanol: 25 mg/ml; Ethanol:PBS(pH 7.2) (1:1): 0.5 mg/ml |
| pka | pKa 5.0 (Uncertain) |
| form | Crystalline Powder, Crystals and/or Chunks |
| color | Off-white to brownish |
| Water Solubility | 5.55 g/100 mL |
| Sensitive | Air & Light Sensitive |
| Merck | 14,474 |
| Stability: | Stable. Incompatible with strong oxidizing agents, strong acids, strong bases. Light sensitive. Degrades under the action of mild oxidizing agents in the presence of moisture or water. |
| CAS DataBase Reference | 58-15-1(CAS DataBase Reference) |
| NIST Chemistry Reference | Aminopyrine(58-15-1) |
| EPA Substance Registry System | Aminopyrine (58-15-1) |
Safety information for Aminopyrine
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Aminopyrine
| InChIKey | RMMXTBMQSGEXHJ-UHFFFAOYSA-N |
Aminopyrine manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran



You may like
-
 4-Dimethylaminoantipyrine (Aminopyrine) extrapure CAS 58-15-1View Details
4-Dimethylaminoantipyrine (Aminopyrine) extrapure CAS 58-15-1View Details
58-15-1 -
 4-Dimethylaminoantipyrine CAS 58-15-1View Details
4-Dimethylaminoantipyrine CAS 58-15-1View Details
58-15-1 -
 Metamizole EP Impurity D 98% (HPLC) CAS 58-15-1View Details
Metamizole EP Impurity D 98% (HPLC) CAS 58-15-1View Details
58-15-1 -
 4-Dimethylaminoantipyrine CAS 58-15-1View Details
4-Dimethylaminoantipyrine CAS 58-15-1View Details
58-15-1 -
 Amino PyrineView Details
Amino PyrineView Details
58-15-1 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
