Ambroxane
Synonym(s):1,5,5,9-Tetramethyl-13-oxatricyclo[8.3.0.04,9]tridecane
- CAS NO.:6790-58-5
- Empirical Formula: C16H28O
- Molecular Weight: 236.39
- MDL number: MFCD00134491
- EINECS: 229-861-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:57
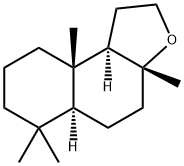
What is Ambroxane?
Description
Ambroxan is a synthetic spice with the special aroma of natural ambergris, and it is recognized as one of the best substitutes for natural ambergris. It is one of the most critical trace components of natural ambergris tincture. Natural ambergris is a precious animal spice. It is a kind of stone in the stomach of sperm whale. It is vomited or excreted on the sea surface by the whale, and it emits a special fragrance in the air for a long time.
The Uses of Ambroxane
(-)-Ambroxide can be used:
To prepare (+)-sclareolide through C-H oxidation strategy.
As a substrate in C(sp3)-H alkylation/arylation studies of ethers.
As a substrate in the study of ethereal hydrocarbon hydroperoxidation using singlet O2.
Definition
ChEBI: Ambroxan is a diterpenoid derived from sclareol that is responsible for the odour of ambergris (a solid, waxy, flammable substance produced in the digestive system of sperm whales). It is an organic heterotricyclic compound and a diterpenoid.
What are the applications of Application
Ambroxan has a strong, characteristic ambergris aroma. Used in high-grade perfumes and cosmetic essences, because it is non-irritating to the human body and non-allergic to animals, it is very suitable for fragrances for skin, hair and fabrics. Often used in soaps, talcum powders, creams and shampoos for perfuming and fixing fragrance.
Preparation
Ambroxene naturally occurring autoxidation or photooxide of the triterpenoid secreted by sperm whales.
Industrially, High acacia acid was prepared from nerolidol. After acid internal esterification, the racemic Ambroxene was obtained.
Ambroxide is synthesized from sclareol, a component of the essential oil of clary sage. Sclareol is oxidatively degraded to a lactone, which is hydrogenated to the corresponding diol. The resulting compound is dehydrated to form ambroxide.
Origin
Ambroxane was first synthesized in the 1950s by the Swiss chemical company Firmenich, and it has since become one of the most widely used aroma compounds in the fragrance industry.
General Description
Ambroxide is a terpenoid, which has vast applications in the perfume industry due to its fixative property and odor. Ambergris, which was originally sourced from sperm whale, has been substituted by synthetic ambroxides.
Flammability and Explosibility
Not classified
Side Effects
The most common side effects associated with Ambroxane use include those which are observed among patients developing allergic reactions. These include pruritus, hives, and swelling in the face, lips, tongue, or throat. It is important to immediately seek medical attention upon the development of dangerous adverse allergic reactions like difficulty in breathing, shortness of breath, convulsions, and mood changes.
Synthesis
Perillyl alcohol is used as raw material to synthesize nordrone ether, which is oxidized by KMnO4 in two steps (Switzerland uses ozone oxidation, Russia uses sodium chromate oxidation). That is (1) alkaline oxidation; (2) weak acid oxidation. The oxide is obtained, and then the oxide is soaped, dehydrated and lactonized to obtain ambroxolide. lactones are reduced to ambroxol with lithium aluminum hydride in ether (or with borane in tetrahydrofuran). D-camphor-β-sulfonic acid is used as a cyclizing agent to cyclize diols to obtain ambrox (foreign cyclizing agents include sulfuric acid, p-toluenesulfonic acid and β-naphthalenesulfonic acid, etc.).
Advantages
One of the key advantages of ambroxan is its stability. Unlike some natural aroma compounds, ambroxan is relatively resistant to oxidation and degradation, which means that it can help to extend the lifespan of a fragrance. It is also very versatile and can be used in a wide range of fragrance types, from fresh and light scents to heavier, more complex fragrances. In addition to its use in perfumes and fragrances, ambroxan is also sometimes used in other products such as detergents, soaps, and other personal care products.
Properties of Ambroxane
| Melting point: | 74-76 °C (lit.) |
| Boiling point: | 273.9±8.0 °C(Predicted) |
| alpha | -30 º (c=1% in toluene) |
| Density | 0.939 |
| vapor pressure | 0.066Pa at 25℃ |
| FEMA | 3471 | 1,5,5,9-TETRAMETHYL-13-OXATRICYCLO(8.3.0.0(4,9))TRIDECANE |
| storage temp. | Sealed in dry,Room Temperature |
| form | powder to crystaline |
| color | White to Almost white |
| Odor | at 1.00 % in dipropylene glycol. ambergris old paper sweet labdanum dry |
| optical activity | [α]20/D 29°, c = 1 in toluene |
| Water Solubility | 1.88mg/L at 20℃ |
| JECFA Number | 1240 |
| CAS DataBase Reference | 6790-58-5(CAS DataBase Reference) |
| NIST Chemistry Reference | 8 «alpha»,12-epoxy-13,14,15,16-tertanorlabdane(6790-58-5) |
| EPA Substance Registry System | Naphtho[2,1-b]furan, dodecahydro-3a,6,6,9a-tetramethyl-, (3aR,5aS,9aS,9bR)- (6790-58-5) |
Safety information for Ambroxane
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Ambroxane
| InChIKey | YPZUZOLGGMJZJO-LQKXBSAESA-N |
Ambroxane manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 Ambroxide, 99% CAS 6790-58-5View Details
Ambroxide, 99% CAS 6790-58-5View Details
6790-58-5 -
 (-)-Ambroxide CAS 6790-58-5View Details
(-)-Ambroxide CAS 6790-58-5View Details
6790-58-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1