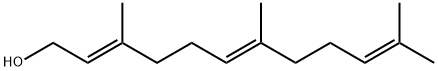
(E,E)-Farnesol
Synonym(s):(E,E)-3,7,11-Trimethyl-2,6,10-dodecatrien-1-ol;trans,trans-3,7,11-Trimethyl-2,6,10-dodecatrien-1-ol
- CAS NO.:106-28-5
- Empirical Formula: C15H26O
- Molecular Weight: 222.37
- MDL number: MFCD00002918
- EINECS: 679-283-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52
What is (E,E)-Farnesol ?
Description
Farnesol is widely present in the flowers, leaves, stems and other parts of plants, especially in some Chinese herbal medicine plants and spice plants with a high content, such as Cinnamomum chinensis, Pinellia sinensis, loquat leaves, etc., which are well developed and utilized An important resource for prospects. Farnesol is one of the important active components of Chinese herbal medicine plants, and also one of the main fragrance components of some important essential oils of spice plants. Therefore, it has been widely used in medicine, pesticides, cosmetics and daily chemicals that require its biological activity.
Chemical properties
Clear Colourless Oil
The Uses of (E,E)-Farnesol
Farnesol is a natural organic compound which is present in many essential oils.
The Uses of (E,E)-Farnesol
trans,trans-Farnesol is an intermediate used in the biological synthesis of cholesterol from mevalonic acid. It is used in the perfume industry. Further, it is used in the preparation of terpenes such as squalenes, cembranoids and forskolin. It is also used in the synthesis of Cecropia juvenile hormone.
Definition
ChEBI: Farnesol is a farnesane sesquiterpenoid that is dodeca-2,6,10-triene substituted by methyl groups at positions 3, 7 and 11 and a hydroxy group at position 1. It has a role as a plant metabolite, a fungal metabolite and an antimicrobial agent. It is a farnesane sesquiterpenoid, a primary alcohol and a polyprenol.
Synthesis Reference(s)
Journal of the American Chemical Society, 102, p. 3298, 1980 DOI: 10.1021/ja00529a091
The Journal of Organic Chemistry, 40, p. 3617, 1975 DOI: 10.1021/jo00912a039
General Description
trans,trans-Farnesol is sesquiterpene alcohol, which is commonly found in many essential oils, citrus fruits, propolis, and plant-derived foods and beverages. It is an effective bioactive agent that can be used in pharmaceuticals and cosmetics.
Purification Methods
The main impurity is the cis-trans isomer. Purify it by gas chromatography using a 4ft x 0.125in 3%OV-1 column at 150o. [Corey et al. J Am Chem Soc 92 6637 1970, Popjak et al. J Biol Chem 237 56 1962.] It has also been fractionated through a 14-in Podbielniak column (p 11) at 11o/0.35mm. Alternatively it has been purified by gas chromatography using SF96 silicone on Fluoropak columns or Carbowax 20M on Fluoropak or base-washed 30:60 firebrick (to avoid decomposition, prepared by treating the firebrick with 5N NaOH in MeOH and washed with MeOH to pH 8) at 210o with Helium carrier gas at 60 mL/min flow rate. The diphenylcarbamoyl derivative has m 61-63o (from MeOH) and has an IR band at 3500 cm-1. [Bates et al. J Org Chem 28 1086 1963, Beilstein 1 IV 2335.]
Properties of (E,E)-Farnesol
| Melting point: | 61-63 °C |
| Boiling point: | 149 °C4 mm Hg(lit.) |
| Density | 0.886 g/mL at 20 °C(lit.) |
| refractive index | n |
| Flash point: | 205 °F |
| storage temp. | Sealed in dry,2-8°C |
| solubility | Chloroform (Sparingly), DMSO (Slightly) Ethyl Acetate (Slightly), Methanol (Spar |
| form | neat |
| pka | 14.42±0.10(Predicted) |
| form | Liquid |
| color | Colourless |
| Odor | at 100.00 %. mild muguet floral sweet lily waxy |
| Water Solubility | Miscible with alcohol. Immiscible with water. |
| Sensitive | Light Sensitive |
| BRN | 1723039 |
| Stability: | Light Sensitive |
| CAS DataBase Reference | 106-28-5(CAS DataBase Reference) |
| NIST Chemistry Reference | 2,6,10-Dodecatrien-1-ol, 3,7,11-trimethyl-, (E,E)-(106-28-5) |
| EPA Substance Registry System | (E,E)-Farnesol (106-28-5) |
Safety information for (E,E)-Farnesol
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H317:Sensitisation, Skin |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P272:Contaminated work clothing should not be allowed out of the workplace. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P333+P313:IF SKIN irritation or rash occurs: Get medical advice/attention. |
Computed Descriptors for (E,E)-Farnesol
| InChIKey | CRDAMVZIKSXKFV-YFVJMOTDSA-N |
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran




You may like
-
 trans,trans-Farnesol CAS 106-28-5View Details
trans,trans-Farnesol CAS 106-28-5View Details
106-28-5 -
 trans,trans-Farnesol 98% (GC) CAS 106-28-5View Details
trans,trans-Farnesol 98% (GC) CAS 106-28-5View Details
106-28-5 -
 trans,trans-Farnesol CAS 106-28-5View Details
trans,trans-Farnesol CAS 106-28-5View Details
106-28-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1