ALUMINUM POTASSIUM SULFATE
- CAS NO.:10043-67-1
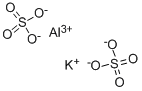
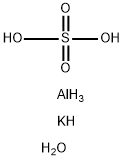
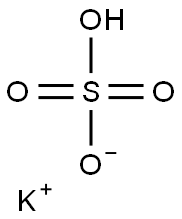
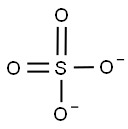
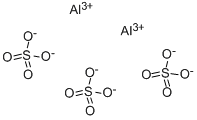
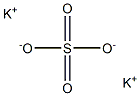
- Empirical Formula: AlKO8S2
- Molecular Weight: 258.21
- MDL number: MFCD00003428
- EINECS: 233-141-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 13:37:16
What is ALUMINUM POTASSIUM SULFATE?
Absorption
Potassium alum is found in its dodecahydrate form that produces a very large molecule. This large molecule cannot be absorbed through the skin when this substance is included as an astringent agent in topical OTC. If ingested, the aluminum salts are rapidly solubilized in the stomach and then they can generate aluminum hydroxide or poorly absorbed basic aluminum salts.
Toxicity
There is no evidence of potassium alum that demonstrates any suspicious for it to be a hazard for the consumers in the currently approved levels of use.
Chemical properties
white powder(s); absorbs atmospheric moisture [MER06]
Chemical properties
The PhEur 6.0 describes potassium alum as a granular powder, or colorless, transparent, crystalline masses. The JP XV describes it as colorless or white crystals or powder. Potassium alum is odorless and has a slightly sweet, strongly astringent taste.
The Uses of ALUMINUM POTASSIUM SULFATE
Astringent (topical).
Indications
Potassium alum is considered safe by the FDA and its use is in homepathic or OTC products. Due to its presence in several different drugs, the main indications for the use of potassium alum are:
-Constipation
-Cosmetic or drug astringent, helping the shrinkage of tissues and the dry of secretions
-Oral health care drug
-Part of formulation in cleansing products, skin-care products, mosturizers, face powders and deodorants
-Antiperspirant
-Antifungal
Background
Potassium alum is considered by the FDA as a generally recognized as safe (GRAS) substance. It is an inorganic salt, also called potassium aluminum sulfate with a formula of AlK(SO4)2 that is predominantly produced in the dodecahydrate form (AlK(SO4)2 * 12H2O). Potassium alum is formed by large, transparent crystals that are used in different products like food or drugs as a buffer, neutralizing or forming agent.
Definition
ChEBI: A metal sulfate composed of potassium, aluminium and sulfate ions in the ration 1:1:2.
Production Methods
Potassium alum is manufactured by treating bauxite with sulfuric acid and then potassium sulfate. Alternatively, aluminum sulfate is reacted with potassium sulfate.
Flammability and Explosibility
Non flammable
Pharmaceutical Applications
Potassium alum precipitates proteins and is a powerful astringent.
The ability to precipitate proteins is utilized in the manufacture of
vaccines, where purified proteins are coprecipitated with and
adsorbed onto potassium alum.
Potassium alum is often included in preparations used as
mouthwashes or gargles and in dermatological preparations, and
it may be used as a topical hemostatic, either as a solid or as a
solution. Intravesical instillation of potassium alum, typically as a
1% solution, has been used for hemorrhagic cystitis.
Pharmacokinetics
The presence of potassium alum reduces swollen mucous membranes that result from inflammation of the nasal, gastrointestinal and urinary passages as well as in the presence of excessive secretions. The induction of the coagulation cascade will also stop bleeding.
Safety
Potassium alum is often included in preparations used as
mouthwashes or gargles and in dermatological preparations.
Large doses of potassium alum act as an irritant and may be
corrosive; gum necrosis and gastrointestinal hemorrhage have
occurred. Acute encephalopathy has been reported following
bladder irrigation with alum solutions in the treatment of bladder
hemorrhage. Anecdotal evidence suggests that this practice should
be avoided for patients with renal insufficiency.
Metabolism
Potassium alum does not go through a metabolic pathway. When ingested or absorbed, it will get rapidly dissolved and it will form ions that will later generate other salt derivatives.
storage
Store in a cool, dry place in tightly closed containers. Stable under normal temperatures and pressures. When kept for a long time at 60–65°C (or over sulfuric acid) potassium alum dodecahydrate loses water, which is reabsorbed on exposure to air. It becomes anhydrous at about 200°C.
Incompatibilities
Potassium alum is incompatible with strong oxidizing agents, aluminum, copper, steel, and zinc. When it is dispensed in powders with phenol, salicylates, or tannic acid, gray or green colors may be developed owing to traces of iron in the alum.
Regulatory Status
GRAS listed. Included in the FDA Inactive Ingredients Database (vaginal; suppository). Included in medicines licensed in the UK.
Properties of ALUMINUM POTASSIUM SULFATE
| Density | 1.725 g/cm3 |
| solubility | Freely soluble in water, very soluble in boiling water;
soluble in glycerol; practically insoluble in ethanol (96%). |
| form | white hygroscopic powder |
| color | white |
| Water Solubility | g/100g H2O: 3.00 (0°C), 5.90 (20°C), 109 (90°C) [LAN05] |
| Merck | 13,359 |
| CAS DataBase Reference | 10043-67-1(CAS DataBase Reference) |
| EPA Substance Registry System | Potassium aluminum sulfate (10043-67-1) |
Safety information for ALUMINUM POTASSIUM SULFATE
Computed Descriptors for ALUMINUM POTASSIUM SULFATE
ALUMINUM POTASSIUM SULFATE manufacturer
JSK Chemicals
ARRAKIS INDUSTRIES LLP
Techno Pharmchem
Zama Chemical
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran

You may like
-
 10043-67-1 ALUMINIUM POTASSIUM SULPHATE 98%View Details
10043-67-1 ALUMINIUM POTASSIUM SULPHATE 98%View Details
10043-67-1 -
 10043-67-1 97%View Details
10043-67-1 97%View Details
10043-67-1 -
 Aluminum potassium sulfate 99%View Details
Aluminum potassium sulfate 99%View Details -
 Aluminum potassium sulfate 98%View Details
Aluminum potassium sulfate 98%View Details -
 ALUMINIUM POTASSIUM SULPHATE 99%View Details
ALUMINIUM POTASSIUM SULPHATE 99%View Details
10043-67-1 -
 Aluminum potassium sulfate 99%View Details
Aluminum potassium sulfate 99%View Details -
 Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
67967-07-1 -
 132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6
