Alpelisib (BYL719)
- CAS NO.:1217486-61-7
- Empirical Formula: C19H22F3N5O2S
- Molecular Weight: 441.47
- MDL number: MFCD22417085
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-14 20:53:18
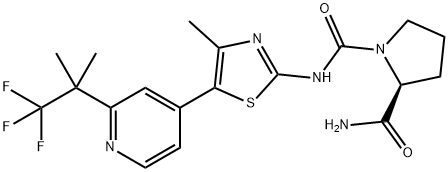
What is Alpelisib (BYL719)?
Absorption
Alpelisib reached a peak concentration in plasma of 1320±912ng/mL after 2 hours. Alpelisib has an AUClast of 11,100±3760h ng/mL and an AUCINF of 11,100±3770h ng/mL. A large, high fat meal increases the AUC by 73% and Cmax by 84% while a small, low fat meal increases the AUC by 77% and Cmax by 145%.
Toxicity
Patients experiencing an overdose may present with hyperglycemia, nausea, asthenia, and rash. There is no antidote for an overdose of alpelisib so patients should be treated symptomatically.
Data regarding an LD50 is not readily available. In clinical trials, patients were given doses of up to 450mg once daily.
Description
BYL719 is an inhibitor of phosphoinositide 3-kinase α (PI3Kα; IC50s = 4.6, 4, and 4.8 nM for wild-type, E545K mutant, and H1047R mutant PI3K, respectively). It is selective for PI3Kα over PI3Kβ, PI3Kδ, PI3Kγ, and PI4Kβ (IC50s = 1,156, 290, 250, and 581 nM, respectively), as well as VPS34, mTOR, DNA-PK, and ATR (IC50s = >9,100 nM for all). BYL719 (12.5, 25, and 50 mg/kg) reduces tumor volume in a PI3Kα-dependent Rat1-myr-p110α mouse xenograft model. It also reduces tumor burden in THP-1 acute myeloid leukemia (AML) and MCF-7 breast cancer mouse xenograft models. Formulations containing BYL719 have been used in the treatment of advanced or metastatic breast cancer.
The Uses of Alpelisib (BYL719)
(2S)-N1-[4-Methyl-5-[2-(2,2,2-trifluoro-1,1-dimethylethyl)-4-pyridinyl]-2-thiazolyl]-1,2-pyrrolidinedicarboxamide is a newly developed phosphatidylinositol-3-kinase (PI3K) inhibitor and a mTOR inhibit or for the treatment of proliferative diseases.
The Uses of Alpelisib (BYL719)
(2S)-N1-[4-Methyl-5-[2-(2,2,2-trifluoro-1,1-dimethylethyl)-4-pyridinyl]-2-thiazolyl]-1,2-pyrrolidinedicarboxamide is a newly developed phosphatidylinositol-3-kinase (PI3K) inhibitor and a mTOR inhibitor for the treatment of proliferative diseases.
What are the applications of Application
BYL719 is a selective inhibitor of phosphoinositide 3-kinase α (PI3Kα), equipotent against wild type and mutant isoforms.
Background
Alpelisib is a phosphatidylinositol 3-kinase (PI3K) inhibitor with potent antitumor activity. It works by selectively inhibiting class I PI3K p110α , which is the catalytic subunit of PI3K, a lipid kinase that plays a role in various biological processes, including proliferation, survival, differentiation, and metabolism. Alpelisib was designed to target this enzyme that appears to be mutated at a rate of nearly 30% in human cancers, leading to hyperactivation.
There are several isoform-specific PI3K inhibitors that are under clinical development or currently approved, such as idelalisib used for chronic lymphocytic leukemia (CLL). Approved by the FDA in May 2019, alpelisib is the first approved PI3K inhibitor indicated for the treatment of hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative, PIK3CA-mutated, advanced or metastatic breast cancer in combination with fulvestrant for postmenopausal women and male patients. To initiate alpelisib therapy, it is required that the presence of a PIK3CA mutation in the tissue and/or liquid biopsy sample collection should be confirmed via FDA-approved diagnostic tests. Alpelisib is marketed under the trade name Piqray and is available as oral tablets. Studies evaluating the therapeutic effectiveness of alpelisib in other cancers, such as ovarian cancer and colorectal cancer , are under ongoing investigations.
Alpelisib was granted FDA approval on 24 May 2019. In April 2022, the FDA granted the use of alpelisib in the treatment of PIK3CA-Related Overgrowth Spectrum (PROS) in adults and children who require systemic therapy.
Indications
Alpelisib is indicated in combination with fulvestrant to treat postmenopausal women, and men, with advanced or metastatic breast cancer. This cancer must be hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative, and PIK3CA- mutated. The cancer must be detected by an FDA-approved test following progression on or after an endocrine-based regimen.
Alpelisib is also used to treat adult and pediatric patients two years of age and older with severe manifestations of PIK3CA-Related Overgrowth Spectrum (PROS) who require systemic therapy. This indication is approved under accelerated approval based on response rate and duration of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial(s).
Definition
ChEBI: (2S)-N1-[4-methyl-5-[2-(1,1,1-trifluoro-2-methylpropan-2-yl)-4-pyridinyl]-2-thiazolyl]pyrrolidine-1,2-dicarboxamide is a proline derivative.
brand name
Piqray
General Description
Class: lipid kinase; Treatment: breast cancer; Other name: NVP-BYL719; Elimination half-life = 13.7 h; Protein binding = 89%
Pharmacokinetics
Alpelisib does not prolong the QTcF interval. Patients taking alpelisib experience a dose dependent benefit from treatment with a 51% advantage of a 200mg daily dose over a 100mg dose and a 22% advantage of 300mg once daily over 150mg twice daily. This suggests patients requiring a lower dose may benefit from twice daily dosing.
Metabolism
Alpelisib is metabolized by hydrolysis reactions to form the primary metabolite. It is also metabolized by CYP3A4. The full metabolism of Alpelisib has yet to be determined but a series of reactions have been proposed. The main metabolic reaction is the substitution of an amine group on alpelisib for a hydroxyl group to form a metabolite known as M4 or BZG791. Alpelisib can also be glucuronidated to form the M1 and M12 metabolites.
References
1. furet p, guagnano v, fairhurst ra et al. discovery of nvp-byl719 a potent and selective phosphatidylinositol-3 kinase alpha inhibitor selected for clinical evaluation. bioorg med chem lett 2013; 23: 3741-3748. 2. azab f, vali s, abraham j et al. pi3kca plays a major role in multiple myeloma and its inhibition with byl719 decreases proliferation, synergizes with other therapies and overcomes stroma-induced resistance. br j haematol 2014; 165: 89-101. 3. juric d, argiles g, burris h et al. phase i study of byl719, an alpha-specific pi3k inhibitor, in patients with pik3ca mutant advanced solid tumors: preliminary efficacy and safety in patients with pik3ca mutant er-positive (er+) metastatic breast cancer (mbc). cancer res 2012; 72: p6-10.
Properties of Alpelisib (BYL719)
| Density | 1.391 |
| storage temp. | Store at -20°C |
| solubility | ≥22.07 mg/mL in DMSO; insoluble in H2O; insoluble in EtOH |
| pka | 6.29±0.70(Predicted) |
| form | White solid. |
| color | Off-white to light yellow |
Safety information for Alpelisib (BYL719)
Computed Descriptors for Alpelisib (BYL719)
| InChIKey | STUWGJZDJHPWGZ-LBPRGKRZSA-N |
| SMILES | N1(C(NC2=NC(C)=C(C3C=CN=C(C(C)(C)C(F)(F)F)C=3)S2)=O)CCC[C@H]1C(N)=O |
New Products
3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-Iodophenylacetic acid 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 5-Amino Salicylicacid, 95% 4-(Cyanomethyl)benzoic acid, 95% High Vac grease Trimethylsilyl cyanide, 90% Phenyl dichlorophosphate,98% Decanoic acid,98% (R)-2-Methylpyrolidine-2-carboxylic acid (De Mepro) Ramipril Sacubitril- Valsartan Boc-his(trt)-OH Fmoc-L-Glu-OtBu Boc-L-Tyr(tBu)-OH 2-Chloromethyl-4-methyl-quinazoline 2-[1-(Mercaptomethyl)Cyclopropyl]Acetic Acid Trans-4-Aminocyclohexanol [4tac] 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] L-Glutamic Acid Diethyl Ester Hydrochloride Bis(2-Chloroethyl) Amine HydrochlorideRelated products of tetrahydrofuran
![4H-PYRIDO[1,2-A]PYRIMIDIN-4-ONE, 7-METHYL-2-(4-MORPHOLINYL)-9-[1-(PHENYLAMINO)ETHYL]-](https://img.chemicalbook.in/CAS/GIF/663619-89-4.gif)


You may like
-
 Alpelisib >95% CAS 1217486-61-7View Details
Alpelisib >95% CAS 1217486-61-7View Details
1217486-61-7 -
 (3S, 4R)-Tert-butyl-3-amino-4-fluoro-pyrrolidine-1- carboxylate 98%View Details
(3S, 4R)-Tert-butyl-3-amino-4-fluoro-pyrrolidine-1- carboxylate 98%View Details
1174020-30-4 -
 570-23-0 98%View Details
570-23-0 98%View Details
570-23-0 -
 1-Chloro-7-methoxy-isoquinoline-6-carbonitrile 98%View Details
1-Chloro-7-methoxy-isoquinoline-6-carbonitrile 98%View Details
1427393-40-5 -
 122775-35-3 3, 4-Dimethoxy phenyl boronic acid 98%View Details
122775-35-3 3, 4-Dimethoxy phenyl boronic acid 98%View Details
122775-35-3 -
 1304494-06-1 98%View Details
1304494-06-1 98%View Details
1304494-06-1 -
 VANOXERINE DIHYDROCHLORIDE 98%View Details
VANOXERINE DIHYDROCHLORIDE 98%View Details
67469-78-7 -
 1936128-86-7 98%View Details
1936128-86-7 98%View Details
1936128-86-7
