D-ERYTHRO-SPHINGOSINE
Synonym(s):Sphingosine;D-erythro-sphingosine;Sphingosine 2S, 3R; D-erythro-Sphingosine; (2S,3R,4E)-2-aminooctadec-4-ene-1,3-diol; (2S,3R,4E)-2-amino-1,3-octadec-4-enediol;(2S,3R,4E)-2-Amino-4-octadecene-1,3-diol;trans-D -erythro-2-Amino-4-octadecene-1,3-diol
- CAS NO.:123-78-4
- Empirical Formula: C18H37NO2
- Molecular Weight: 299.49
- MDL number: MFCD00036751
- EINECS: 204-651-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 12:07:08
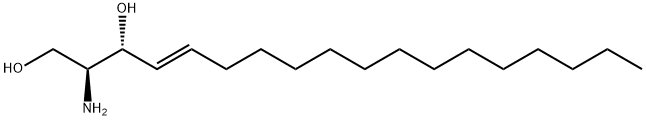
What is D-ERYTHRO-SPHINGOSINE?
Description
Sphingosine (123-78-4) is an inhibitor of protein kinase C (IC50 = 1-3 μM).1 It is abundant in cell membranes and is an important mediator in various biochemical pathways.2-4
Chemical properties
White Crystalline Solid
The Uses of D-ERYTHRO-SPHINGOSINE
D-erythro-Sphingosine is used to inhibits protein kinase C and calmodulin-dependent enzymes, induces apoptosis. And also used to constituent of cell membranes.
The Uses of D-ERYTHRO-SPHINGOSINE
Selective inhibitor of protein kinase C activity and phorbol dibutyrate binding in vitro in human platelets; does not inhibit protein kinase A or myosin light chain kinase; inhibits calmodulin-dependent enzymes; natural isomer of sphingosine
The Uses of D-ERYTHRO-SPHINGOSINE
Sphingosine (d18:1) has been used:
- for sphingosine inhalations studies in mice to evaluate its therapeutic potential in respiratory epithelial cells
- to investigate its effect on Pseudomonas aeruginosa strains
- as a standard in liquid chromatography tandem mass spectrophotometry for the quantification of epidermal ceramides
What are the applications of Application
D-erythro-Sphingosine is a potent, highly selective PKC inhibitor
Definition
cerebroside: Any one of a class ofglycolipids in which a single sugarunit is bound to a sphingolipid. The most commoncerebrosides are galactocerebrosides,containing the sugar group galactose;they are found in the plasma membranesof neural tissue and are abundantin the myelin sheaths ofneurones.
General Description
Sphingosine (d18:1) is a spingoid base present in plant and animals. It has 2-amino-1,3-diol functionality and its extraction from animal tissue is laborious. Sphingosine (d18:1) synthesis is performed by chirospecific method using D-galactose.
Biological Activity
Inhibitor of protein kinase C and calmodulin-dependent enzymes, but may stimulate mast cells by activation of protein kinase C.
Biochem/physiol Actions
A constituent of cell membranes. Precursor of ceramide. Selective inhibitor of protein kinase C, but does not inhibit protein kinase?A or myosin light chain kinase. Inhibitor of calmodulin-dependent enzymes.
Purification Methods
D-Sphingosine is purified by recrystallisation from EtOAc, Et2O or pet ether (60-80o). It is insoluble in H2O but is soluble in Me2CO, EtOH and MeOH. It has IR bands at 1590 and 875 cm-1, and is characterised as the tribenzoate m 122-123o (from 95% EtOH). [Tipton Biochemical Preparations 9 127 1962.]
References
1) Merrill et al., (1989) Structural requirements for long-chain (sphingoid) base inhibition of protein kinase C in vitro and for the cellular effects of these compounds; Biochemistry, 28 3138 2) Ohanian and Ohanian (2001) Sphingolipids in mammalian cell signaling; Cell. Mol. Life Science, 58 2053 3) Ruvolo (2003) Intracellular signal transduction pathways activated by ceramide and its metabolites; Pharm. Res., 47 383 4) Olivier (2002) Sphingosine in apoptosis signaling; Biochim. Biophys. Acta, 1585 153
Properties of D-ERYTHRO-SPHINGOSINE
| Melting point: | 74.2-78.1 °C |
| Boiling point: | 440.8°C (rough estimate) |
| Density | 0.9758 (rough estimate) |
| refractive index | 1.5000 (estimate) |
| storage temp. | -20°C |
| solubility | chloroform: 20 mg/mL, clear, colorless to very faintly yellow |
| form | Powder or Waxy Solid |
| pka | 11.80±0.45(Predicted) |
| color | White to cream |
| Merck | 14,8747 |
| BRN | 1727294 |
| Stability: | Stable. Incompatible with strong acids, strong bases, strong oxidizing agents. |
| CAS DataBase Reference | 123-78-4 |
Safety information for D-ERYTHRO-SPHINGOSINE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for D-ERYTHRO-SPHINGOSINE
| InChIKey | WWUZIQQURGPMPG-KRWOKUGFSA-N |
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
![N-LAUROYL-D-ERYTHRO SPHINGOSINE [LAUROYL-1-14C]](https://img.chemicalbook.in/StructureFile/ChemBookStructure4/GIF/CB0704612.gif)

![SPHINGOSINE, D-ERYTHRO [3-3H]-1-PHOSPHATE](https://img.chemicalbook.in/StructureFile/ChemBookStructure4/GIF/CB3371192.gif)
![N-OCTANOYL-D-ERYTHRO-DIHYDROSPHINGOSINE [4,5-3H] 1-PHOSPHATE](https://img.chemicalbook.in/StructureFile/ChemBookStructure4/GIF/CB8668493.gif)


You may like
-
 D-Sphingosine CAS 123-78-4View Details
D-Sphingosine CAS 123-78-4View Details
123-78-4 -
 Sphingosine (d18:1) CAS 123-78-4View Details
Sphingosine (d18:1) CAS 123-78-4View Details
123-78-4 -
 D-Sphingosine CAS 123-78-4View Details
D-Sphingosine CAS 123-78-4View Details
123-78-4 -
 D-erythro-Sphingosine, Free Base, High Purity CAS 123-78-4View Details
D-erythro-Sphingosine, Free Base, High Purity CAS 123-78-4View Details
123-78-4 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4
