Alfuzosin
- CAS NO.:81403-80-7
- Empirical Formula: C19H27N5O4
- Molecular Weight: 389.45
- MDL number: MFCD00865792
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-11 20:33:26
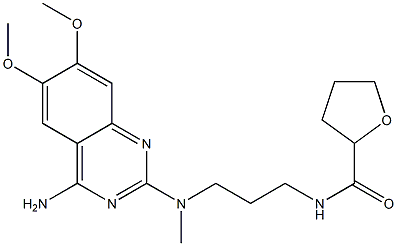
What is Alfuzosin?
Absorption
Alfuzosin is readily absorbed in the gastrointestinal tract and the absolute bioavailability under fed conditions is 49%. In patients over 75 years of age, alfuzosin is absorbed more rapidly and peak plasma levels are higher. One source mentions a bioavailability of 64%. After multiple doses under fed conditions, Cmax is achieved in 8 hours. Cmax and AUC0-24 values are about 13.6 ng/mL and 194 ng·h/mL, respectively. Steady-state plasma concentrations are achieved after the second dose and are 1.2 to 1.6 times higher than after a single dose. With the extended-release formulation, alfuzosin release is sustained over 20 hours with a rate of dissolution ranging between 2 and 12 hours.
Toxicity
The oral LD50 of alfuzosin is 2300 mg/kg in male mice and 1950 mg/kg in female mice. An overdose of alfuzosin can cause hypotension. Cardiovascular support should be initiated immediately. The patient should be kept in the supine position to aid in restoring pressure and managing heart rate. Fluid resuscitation should also be considered in severe cases; sometimes, vasopressors are required. Renal function should be monitored frequently. Dialysis may not be of benefit to alfuzosin protein binding of up to 90%.
Description
Alfuzosin hydrochloride is a post-synaptic a-antagonist useful in the symptomatic treatment of benign prostatic hypertrophy and acute adenoma by lowering urethral contractions induced by alpha, stimulation. Alfuzosin is also being developed as an antihypertensive; its efficacy in this indication is comparable to prazosin with no significant change in heart rate.
Originator
Synthelabo (France)
The Uses of Alfuzosin
dietary supplement
Indications
Alfuzosin is used to treat the signs and symptoms of benign prostatic hyperplasia (BPH).
Background
Benign prostatic hyperplasia (BPH) refers to a benign growth or hyperplasia of the prostate and leads to lower urinary tract symptoms in men, such as urgency, frequency and changes to urine flow. The prevalence of BPH is as high as 50%-60% for males in their 60's, and this prevalence increases to 80%-90% of those over 70. Alfuzosin is an alpha-1 adrenergic blocker used in the symptomatic treatment of BPH that works by relaxing the muscles in the prostate and bladder neck. It was initially approved by the FDA in 2003 and is marketed by several pharmaceutical companies.
Definition
ChEBI: Alfuzosin is a monocarboxylic acid amide, a tetrahydrofuranol and a member of quinazolines. It has a role as an antineoplastic agent, an antihypertensive agent and an alpha-adrenergic antagonist.
brand name
Uroxatral (Sanofi Aventis).
General Description
Alfuzosin is also a quinazoline 1-blockerbut differs from terazosin in replacing the piperazine ring interazosin with an open piperazine ring (a rotatable propylenediaminegroup). Alfuzosin is more selective for thesubtype of α1A-receptor in the prostate gland than those invascular tissue. Thus, it has been used extensively in treatingBPH as a first-line drug with fewer cardiovascular sideeffects than terazosin and doxazosin.
Pharmacokinetics
By selectively inhibiting alpha adrenergic receptors in the lower urinary tract, alfuzosin causes smooth muscle relaxation in the bladder neck and prostate, improving urine flow, thereby reducing BPH symptoms. Additionally, alfuzosin reduces the vasoconstrictor effect of catecholamines (epinephrine and norepinephrine), leading to peripheral vasodilation. This leads to a risk of postural hypotension/syncope, and prescribing information warns that caution should be exercised in patients who take nitrates, antihypertensives, or have experienced decreased blood pressure after using other medications.
Metabolism
Alfuzosin undergoes extensive hepatic metabolism; only 11% of the administered dose is detected unchanged in the urine. Alfuzosin is metabolism occurs via three metabolic pathways: oxidation, O-demethylations, and N-dealkylation. Metabolites of alfuzosin are not pharmacologically active and CYP3A4 is main hepatic cytochrome enzyme responsible for its metabolism.
Properties of Alfuzosin
| Melting point: | 178-180 °C |
| Density | 1.272±0.06 g/cm3(Predicted) |
| storage temp. | -20°C Freezer |
| solubility | DMSO (Sparingly), Methanol (Slightly) |
| form | Solid |
| pka | 14.80±0.20(Predicted) |
| color | White to Off-White |
| CAS DataBase Reference | 81403-80-7(CAS DataBase Reference) |
Safety information for Alfuzosin
Computed Descriptors for Alfuzosin
Alfuzosin manufacturer
KPS Chemicals And Pharmaceuticals
Aspen Biopharma Labs Pvt Ltd
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 81403-80-7 ALFUZOSINView Details
81403-80-7 ALFUZOSINView Details
81403-80-7 -
 Alfuzosin 98%View Details
Alfuzosin 98%View Details -
 Alfuzosin 98%View Details
Alfuzosin 98%View Details -
 Alfuzosin 81403-80-7 98%View Details
Alfuzosin 81403-80-7 98%View Details
81403-80-7 -
 Alfuzosin 81403-80-7 98%View Details
Alfuzosin 81403-80-7 98%View Details
81403-80-7 -
 Alfuzosin 81403-80-7 98%View Details
Alfuzosin 81403-80-7 98%View Details
81403-80-7 -
 Alfuzosin 98.00% CAS 81403-80-7View Details
Alfuzosin 98.00% CAS 81403-80-7View Details
81403-80-7 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1