Alendronate sodium
- CAS NO.:129318-43-0
- Empirical Formula: C4H13NNaO7P2
- Molecular Weight: 272.09
- MDL number: MFCD01861681
- EINECS: 603-329-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-06-13 18:20:42

What is Alendronate sodium?
Description
Alendronate sodium is the fourth bisphosphonate to reach the market for the treatment of postmenopausal osteoporosis. Bisphosphonates are potent inhibitors of bone resorption. They reduce pain and complications due to bone metastases, are effective in Paget's disease and in increasing bone mineral density. These agents bind tightly to hydroxyapatite crystals and are retained on bone resorption surfaces. Local release of the bisphosphonates occurs by acidification during the process of the bone resorption to impair the osteoclasts' ability to resorb bone. Alendronate is more potent than other bisphosphonates such as clodronate, pamidronate, and etidronate and is reported to have no deleterious effects on bone. It has also been shown to reduce hypercalcemia in cancer patients.
Originator
Istituto Gentili (Italy)
Manufacturing Process
4-Amino-1-hydroxybutylidene-1,1-diphosphonic acid (ABDT).
Orthosphophorous acid (102.7 g; 1.25 moles) is introduced into a 2 liter-flask
with condenser, stirrer and dropping funnel, placed on a thermostatized bath;
the air is then removed with a nitrogen stream which is continued during all
the reaction. The acid is melted by heating the bath to 95°C. When melting is
complete, 4-aminobutyric acid (103.3; 1 mole) is added under stirring which
is continued till obtaining a doughy fluid. Phosphorous trihalide (176 ml; 2
moles) is added dropwise causing the mixture to boil and evolution of gaseous
hydrochloric acid which is damped by means of a suitable trap. The addition
rate is adjusted so as to keep a constant reflux for about 60 minutes. When
the addition is nearly over, the mixture swells, slowly hardening. Stirring is
continued as long as possible, whereafter the mixture is heated for further 3
hours. Without cooling, but removing the bath, water (300 ml) is added, first
slowly and then quickly. Heating and stirring are started again. Decolorizing
charcoal is added and the mixture is boiled for about 5 minutes, then hotfiltered
on paper and the filtrate is refluxed for 6 hours. After cooling is slowly
poured in stirred methanol (1500 ml) causing thereby the separation of a
white solid which collected and dried (161 g; 64.6%). The structure of ABDT
is confirmed by IR spectrum, proton magnetic and nuclear magnetic
resonance spectrum and elemental analysis.
The sodium salt of this acid may be prepared by adding of equivalent of
sodium hydroxide.
brand name
Alendros
Therapeutic Function
Antiosteoporotic
Pharmacokinetics
The second-generation agent alendronate sodium was the first bisphosphonate agent approved by the U.S. FDA for the prevention and treatment of osteoporosis and Paget's disease of the bone and is 1,000-fold more potent than etidronate. This derivative, when dosed continuously (5–10 mg/day for osteoporosis and 40 mg/day for Paget's disease) and given with oral calcium supplements (500 mg/day), produced well-mineralized bone and significantly improved BMD (7% in the spine and 4% in the hip) within 18 months. In addition, the vertebral fracture rate was shown to decrease by 47%. A side effect associated with alendronate, chemical esophagitis, has been attributed to inadequate intake of water and lying down after taking the medication. Specific patient instructions were developed to limit the incidence of upper gastrointestinal problems and include: 1) taking the medication with 6 to 8 ounces of water on arising in the morning, 2)remaining in an upright position for at least 30 minutes after taking the medication, and 3) delaying drinking other liquids/eating for at least 30 minutes, if not 1 to 2 hours, to allow maximal absorption of the agent. To enhance absorption, calcium supplements and any aluminum- or magnesium-containing antacids should be dosed separately from the agents in this class.
Clinical Use
The second-generation agent alendronate sodium was the first bisphosphonate agent approved by the U.S. FDA for the prevention and treatment of osteoporosis and Paget's disease of the bone and is 1,000-fold more potent than etidronate.
Properties of Alendronate sodium
| Melting point: | 119 °C |
| storage temp. | Desiccate at -20°C |
| solubility | ≥ 15mg/mL in Water with gentle warming |
| CAS DataBase Reference | 129318-43-0(CAS DataBase Reference) |
Safety information for Alendronate sodium
Computed Descriptors for Alendronate sodium
Abamectin manufacturer
BDR Pharmaceuticals International Pvt Ltd
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran


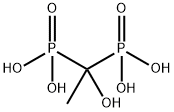



You may like
-
 Alendronate 98%View Details
Alendronate 98%View Details -
 Alendronate sodium 129318-43-0 98%View Details
Alendronate sodium 129318-43-0 98%View Details
129318-43-0 -
 Alendronate sodium 129318-43-0 99%View Details
Alendronate sodium 129318-43-0 99%View Details
129318-43-0 -
 Alendronate sodium 98%View Details
Alendronate sodium 98%View Details
129318-43-0 -
 129318-43-0 99%View Details
129318-43-0 99%View Details
129318-43-0 -
 129318-43-0 Alendronate sodium 98%View Details
129318-43-0 Alendronate sodium 98%View Details
129318-43-0 -
 Alendronate sodium 95% CAS 129318-43-0View Details
Alendronate sodium 95% CAS 129318-43-0View Details
129318-43-0 -
 Alendronate sodium 99%View Details
Alendronate sodium 99%View Details
129318-43-0