Ibandronate sodium monohydrate
Synonym(s):(1-Hydroxy-3-(methylpentylamino)propylidene)bisphosphonic acid sodium;Bondronat
- CAS NO.:138926-19-9
- Empirical Formula: C9H24NNaO8P2
- Molecular Weight: 359.23
- MDL number: MFCD00912167
- EINECS: 639-757-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-16 16:15:04

What is Ibandronate sodium monohydrate?
Description
Ibandronate Sodium Monohydrate is a highly potent nitrogen-containing bisphosphonate used for the treatment of osteoporosis.
Ibandronate Sodium Monohydrate inhibits bone resorption as a sodium salt and complexed with technetium Tc 99m for bone imaging. The monophosphonates are not active and the biphosphonates are used in disorders affecting the skeleton such as metastatic disease, asteoporosis and Paget's. Ibandronate Sodium Monohydrate is an inhibitor of FDPS.
Chemical properties
[1-Hydroxy-3-(methylpentylamino)-propylidene]bisphosphonic acid sodium salt is White Crystalline Powder
The Uses of Ibandronate sodium monohydrate
Inhibits bone resorption as a sodium salt and complexed with technetium Tc 99m for bone imaging. The monophosphonates are not active. Biphosphonates are used in disorders affecting the skeleton such as asteoporosis, metastatic disease, and Paget d
The Uses of Ibandronate sodium monohydrate
bone resorption inhibitor, anthypercalcemic
The Uses of Ibandronate sodium monohydrate
An inhibitor of bone resorption
What are the applications of Application
Ibandronate Sodium Monohydrate is an inhibitor of bone resorption
Mechanism of action
The action of ibandronate on bone tissue is based on its affinity for hydroxyapatite, which is part of the mineral matrix of bone. Ibandronate inhibits osteoclast activity and reduces bone resorption and turnover. In postmenopausal women, it reduces the elevated rate of bone turnover, leading to, on average, a net gain in bone mass.
Pharmacokinetics
Absorption
The absorption of oral ibandronate occurs in the upper gastrointestinal tract. Plasma concentrations increase in a dose-linear manner up to 50 mg oral intake and increases nonlinearly above this dose.
Following oral dosing, the time to maximum observed plasma ibandronate concentrations ranged from 0.5 to 2 hours (median 1 hour) in fasted healthy postmenopausal women. The mean oral bioavailability of 2.5 mg ibandronate was about 0.6% compared to intravenous dosing. The extent of absorption is impaired by food or beverages (other than plain water). The oral bioavailability of ibandronate is reduced by about 90% when BONIVA is administered concomitantly with a standard breakfast in comparison with bioavailability observed in fasted subjects. There is no meaningful reduction in bioavailability when ibandronate is taken at least 60 minutes before a meal. However, both bioavailability and the effect on bone mineral density (BMD) are reduced when food or beverages are taken less than 60 minutes following an ibandronate dose.
Distribution
After absorption, ibandronate either rapidly binds to bone or is excreted into urine. In humans, the apparent terminal volume of distribution is at least 90 L, and the amount of dose removed from the circulation via the bone is estimated to be 40% to 50% of the circulating dose. In vitro protein binding in human serum was 99.5% to 90.9% over an ibandronate concentration range of 2 to 10 ng/mL in one study and approximately 85.7% over a concentration range of 0.5 to 10 ng/mL in another study.
Metabolism
There is no evidence that ibandronate is metabolized in humans.
https://www.accessdata.fda.gov
References
1) Russell (2006), Ibandronate: Pharmacology and preclinical studies; Bone 38 S7
Properties of Ibandronate sodium monohydrate
| Melting point: | 840C (dec) |
| storage temp. | Keep in dark place,Inert atmosphere,2-8°C |
| solubility | Soluble in DMSO (up to at least 25 mg/ml) |
| form | solid |
| color | White |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 3 months. |
| CAS DataBase Reference | 138926-19-9(CAS DataBase Reference) |
Safety information for Ibandronate sodium monohydrate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Ibandronate sodium monohydrate
Ibandronate sodium monohydrate manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

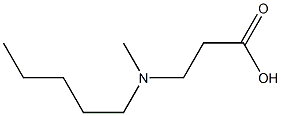
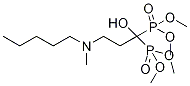
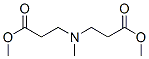
You may like
-
 138926-19-9 Ibandronate Sodium 98%View Details
138926-19-9 Ibandronate Sodium 98%View Details
138926-19-9 -
 138926-19-9 98%View Details
138926-19-9 98%View Details
138926-19-9 -
 Ibandronate sodium monohydrate 98%View Details
Ibandronate sodium monohydrate 98%View Details
138926-19-9 -
 138926-19-9 Ibandronate sodium monohydrate 98%View Details
138926-19-9 Ibandronate sodium monohydrate 98%View Details
138926-19-9 -
 138926-19-9 98%View Details
138926-19-9 98%View Details
138926-19-9 -
 Ibandronate Sodium 99%View Details
Ibandronate Sodium 99%View Details -
 Ibandronate sodium monohydrate CAS 138926-19-9View Details
Ibandronate sodium monohydrate CAS 138926-19-9View Details
138926-19-9 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0
